a 78.0 g sample of gold at 175â°c is put in a foam cup calorimeter with 25.0 g of water at 25.0â°c.…
Published 10 months ago • No plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 5:05
5:05
a 40.0 g sample of glass is put into a calorimeter that contains 250.0 g of water. the glass sample
-
 10:37
10:37
5.22 | a 70.0-g piece of metal at 80.0 °c is placed in 100 g of water at 22.0 °c contained in a
-
 5:01
5:01
thermochemistry: calorimetry- cooling a sample of gold
-
 7:08
7:08
calorimeter
-
 10:25
10:25
how to solve basic calorimetry problems in chemistry
-
 9:24
9:24
calorimetry (constant-pressure calorimeter)
-
 8:03
8:03
complex calorimetry ii
-
 6:10
6:10
calorimetry 02 | problem | calc final temp (genchem)
-
 8:57
8:57
coffee cup calorimetry - specific heat capacity of metal 001
-
 4:14
4:14
heat capacity, specific heat, and calorimetry
-
 6:22
6:22
bomb calorimetry find the temperature change
-
 27:37
27:37
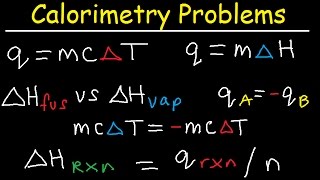
calorimetry problems, thermochemistry practice, specific heat capacity, enthalpy fusion, chemistry
-
 6:09
6:09
⚗️ measuring change in enthalpy in a coffee-cup calorimeter (question 1)
-
 3:43
3:43
104 g of water at 30^@c is taken in a calorimeter made of copper of mass 42 g. when a certain ma...
-
 9:10
9:10
calorimetry: general chemistry lab 10
-
 11:24
11:24
chem 30 calorimetry sample problems
-
 5:05
5:05
calorimetry aluminum cup, water, lead
-
 7:11
7:11
5.24 | a 0.500-g sample of kcl is added to 50.0 g of water in a calorimeter (figure 5.12). if the
-
 0:33
0:33
an aluminum calorimeter with a mass of 100 g contains 250 g of water. the calorim…