(a) verify equation 36.8 by considering a single-electron atom with nuclear charge z e instead of e…
Published 9 days ago • No plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 0:33
0:33
(a) verify equation 36.8 by considering a single-electron atom with nuclear charge ze inst…
-
 4:31
4:31
a single electron ion has nuclear charge \( z e \) where \( z \) i...
-
 5:26
5:26
a single electron ion has nuclear charge \( z e \) where \( z \) i...
-
 3:25
3:25
a single electron ion has nuclear chrage ` ze` where `z` is atomic number
-
 7:14
7:14
how to calculate the effective nuclear charge of an electron
-
 30:31
30:31
the basic structure of the atom | chemistry and our universe: how it all works
-
 5:35
5:35
a better way to picture atoms
-
 18:43
18:43
the hydrogen atom
-
 3:23
3:23
a single electron ion has nuclear chrage ` ze` where `z` is atomic number
-
 10:27
10:27
lecture 4 shielding
-
 10:11
10:11
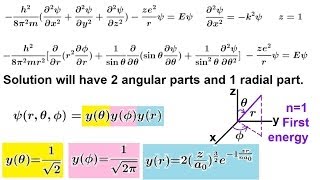
chemistry - electron structures in atoms (23 of 40) the schrodinger eq and the hydrogen atom
-
 9:31
9:31
trick for slater's rule, calculation of screening constant and effective nuclear charge.
-
 0:44
0:44
how much does a physics researcher make?
-
 8:58
8:58
quantum mechanical model (part 8 of 9) - electron shielding and effective nuclear charge
-
 12:30
12:30
how to use slater's rule to estimate the effective nuclear charge
-
 2:25
2:25
a single electron atom has nuclear charge \( z \), where \( z \) is atomic number and \( e \) i...
-
 0:33
0:33
for an ion with nuclear charge z and a single electron, the electric potential energy is -z…
-
 8:21
8:21
example of effective nuclear charge
-
 3:19
3:19
a single electron beam, atom has nuclear charge \( z e \) where \(...
-
 4:41
4:41
the ionization energy for a 1 electron in a silver atom is a determine an approximate value for for
-
 3:47
3:47
bohr's model of an atom|| animated explanation in hinglish || atom and nuclei || physics 12th class
-
 15:52
15:52
how to determine effective nuclear charge using slater's rules