for the cell (at 298k) `ag(s) | agcl(s) | cl^(-)(aq)|| agno_(3)(aq) | ag(s)` which
Published 4 years ago • 421 plays • Length 3:14Download video MP4
Download video MP3
Similar videos
-
 10:56
10:56
cell potential problems - electrochemistry
-
 0:33
0:33
calculate the theoretical yield of agcl starting with 156 ml of 0.75 m nacl. nacl(aq) agno3(aq) -…
-
 5:51
5:51
use tabulated electrode potentials to calculate grxn for each reaction at 25 c a 2 fe3 aq 3 sns
-
 0:33
0:33
when solutions containing silver ions and chloride ions are mixed, silver chloride precipitates …
-
 0:33
0:33
calculate the value of e?cell for the cell reaction: 2au(s) 3ca2 (aq) - 2au3 (aq) 3ca(s) stand…
-
 0:33
0:33
for each of the following cells, write a balanced cell redox equation and calculate eâ°. 1. ag(s) |…
-
 6:41
6:41
tutorial 28-how to maintain ag/agcl reference electrode
-
 0:33
0:33
for the reaction 2co3 (aq) 2cl?(aq)?2co2 (aq) cl2(g). e?=0.483 v what is the cell potential at 25 ?…
-
 1:27:17
1:27:17
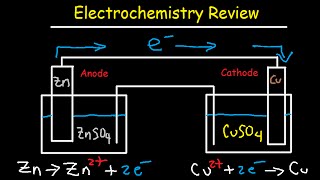
electrochemistry review - cell potential & notation, redox half reactions, nernst equation
-
 11:37
11:37
how to mix and spread hard carbon electrodes
-
 8:36
8:36
tutorial 01- how to clean ag/agcl reference electrode ii how to maintain ag/agcl reference electrode
-
 2:48
2:48
std potential zn(s) cu2 (aq) gives zn2 (aq) cu(s) 1.1 v. calculate std gibbs energy change.
-
 0:33
0:33
calculate the emf of the following zn-ag cell at 22.3â°c. if the concentration of znso4 and agno3 a…
-
 0:26
0:26
the standard electrode potential for daniell cell is 1.1v. calculate the standard gibbs energy f....
-
 0:33
0:33
calculate the cell potential for the galvanic cell in which the reaction: fe(s) au3 (aq) → fe3 …
-
 3:22
3:22
the reaction `1//2hg(g) agcl(s) rarr h^(o )(aq) cl^(c-)(aq) ag(s) ` occurs in the galvanic cell.
-
 0:33
0:33
calculate î”î”g, in kj, for the cell below with a voltage of 2.251 v. m | m^2 || h | h2
-
 5:03
5:03
a sample of caco3 and mgco3 weighed 2.21 g s ignited to constant weight of 1.152 g. the composition
-
 0:33
0:33
the density of solid agcl is 5.56 g/cc. the solid is made up of a cubic array of alternate ag^ an…
-
 27:14
27:14
19.4 how to calculate standard cell potential | general chemistry
-
 5:32
5:32
use tabulated electrode potentials to calculate grxn for each reaction at 25 c a pb2 aq mgs pbs
-
 0:33
0:33
the following sketch is of a voltaic cell consisting of two standard electrodes for two metals, m a…