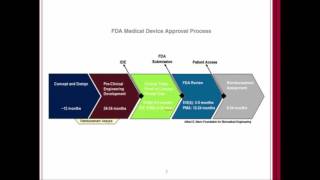
alertwatch: our fda 510k journey
Published 10 years ago • 595 plays • Length 27:09Download video MP4
Download video MP3
Similar videos
-
 28:22
28:22
innovator implications - fda regulation of medical device software (part 3 of 3)
-
 8:02
8:02
commercialization milestones - devices & diagnostics
-
 16:47
16:47
fda regulation of medical devices (part 1 of 3)
-
 1:01:50
1:01:50
unique considerations and the clinical utility of medical devices and diagnostic technologies
-
 15:54
15:54
fda regulation of medical devices and software/apps
-
 10:39
10:39
commercialization milestones - digital health technologies
-
 28:21
28:21
fda regulations and medical device pathways to market
-
 6:00
6:00
ac519 first use and software operation guide
-
 5:39
5:39
propellerads review 2024 | how to run ads on propellerads | $50 sign up bonus
-
 1:29:25
1:29:25
medical device sales strategies
-
 1:03:39
1:03:39
fda regulation of cardiovascular medical devices
-
 32:06
32:06
the fda 510k program
-
 0:21
0:21
oracheck & patient education
-
 0:16
0:16
510(k) tip - schedule your urra and the hf protocol before design freeze
-
 2:39
2:39
introducing radar healthcare
-
 0:42
0:42
how does radara work?
-
 1:15
1:15
dataxplorer – the preconfigured data storage
-
 4:07
4:07
ideal 30 fully automatic molecular diagnostic system regular maintenance
-
 5:13
5:13
welcome to radar healthcare
-
 55:20
55:20
fda 510k premarket notification webinar
-
 0:22
0:22
launch of 5-star ratings system (teaser)