at 25°c, 12 moles of a gas in a 1.2-l container exert a pressure of 150 atm. is this an ideal gas?
Published 3 years ago • 139 plays • Length 3:44Download video MP4
Download video MP3
Similar videos
-
 3:54
3:54
ideal gas properties (example)
-
 5:28
5:28
ideal gas law - practice - 1
-
 0:18
0:18
feeling the pressure of the ideal gas law
-
 5:45
5:45
chemistry: a 500 l container of a gas is at 10 atm and 200 degrees c...
-
 0:58
0:58
ideal gas law how to guide #chemistry #homework #science #shorts #education #youtubeshorts
-
 18:17
18:17
chemistry revision ix - gases
-
 7:01
7:01
ideal gas laws 3: variations of the ideal gas equation
-
 7:00
7:00
a sealed test tube traps 25 0 cm of air at a pressure of 1 00 atm and temperature of 18c the test t
-
 3:01
3:01
lesson 12 - the ideal gas law, part 3 (chemistry tutor)
-
 9:03
9:03
the ideal gas law: crash course chemistry #12
-
 7:35
7:35
general chemistry - properties of gases - partial pressures
-
 4:43
4:43
ideal gas law example # 3
-
 26:31
26:31
5.02 and 5.03 - ideal gas law and dalton's law concepts
-
 1:17:10
1:17:10
gases - chemistry 101
-
 19:24
19:24
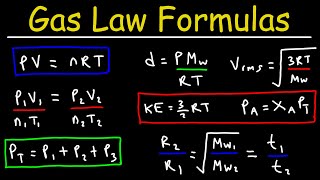
gas law formulas and equations - college chemistry study guide
-
 10:48
10:48
explaining the gas laws in chemistry - volume, temperature, pressure, moles....made easy
-
 4:49
4:49
solving ideal gas law problems | calculate number of moles (part 2, q1)
-
 4:21
4:21
consider two gases, a and b, each in a 1 0 l container with both gases at the same temperature and p
-
 38:35
38:35
mole fractions, ideal gas law, density, molar mass, stoichiometry