balance the equations by oxidation number method \[ \mathrm{zn} \mathrm{hno}_{3} \longrightarrow...
Published 1 year ago • 3.8K plays • Length 3:54Download video MP4
Download video MP3
Similar videos
-
 2:02
2:02
balance the equations by oxidation number method \[ \mathrm{i}_{2} \mathrm{hno}_{3} \longrightar...
-
 3:35
3:35
balance the equation by oxidation number method \( \mathrm{kclo}_{3} \mathrm{s} \longrightarrow ...
-
 3:35
3:35
balance the following redox equations: \[ \mathrm{zn} \mathrm{hno}_{3} \longrightarrow \mathrm{z...
-
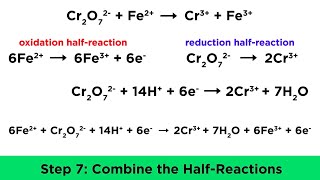 7:31
7:31
balancing redox reactions in acidic and basic conditions
-
 3:35
3:35
balancing a redox reaction / oxidation number method
-
 8:45
8:45
balancing redox reactions by oxidation number method
-
 15:25
15:25
how to calculate oxidation number practice problems
-
 12:39
12:39
balancing redox reactions by ion electron method | easy trick
-
 10:12
10:12
balance the \( \quad \) equations in acidic medium by both oxidation number and ion electron met...
-
 11:27
11:27
balancing redox reactions using oxidation number method
-
![[wow] redox reaction between iron and copper ions #shorts](https://i.ytimg.com/vi/5vtbnGgQCIo/mqdefault.jpg) 0:16
0:16
[wow] redox reaction between iron and copper ions #shorts
-
 3:59
3:59
balance the following reaction by oxidation number method: \[ \math...
-
 34:06
34:06
how to balance redox reactions - general chemistry practice test / exam review
-
 3:23
3:23
balance the following equation by oxidation number method `zn hno_(3) to zn(no_(3))_(2) nh_(4)no_(3)
-
 3:07
3:07
wcln - chemistry - redox -balancing in basic solution using oxidation number method
-
 11:32
11:32
balance the equations in acidic medium by both oxidation number and ion electron methods and ide...
-
 3:54
3:54
the reaction, \[ 3 \mathrm{clo}^{-}(a q) \longrightarrow \mathrm{clo}_{3}^{-}(a q) 2 \mathrm{cl}...
-
 4:33
4:33
( cu hno3 → cu(no3)2 no2 h2o ) balancing redox equations using the oxidation number method
-
 0:06
0:06
find oxidation number shorts tricks #oxidationnumber#oxidationstate#shortfeed
-
 0:59
0:59
what is oxidation number of cr in k2cr2o7? #dubai #chemistry #iitjee #neet #usa #india#cbse#science
-
 1:45
1:45
complete and balance the following equation :zn hno_3 (hot and conc.)to | 10 | study of compo...
-
 3:55
3:55
the oxidation states of \( \mathrm{n} \) in aniline and nitrobenzen...