calculate the molar mass of each of the following compounds. you may round atomic masses to one dec…
Published 1 year ago • 2 plays • Length 0:33
Download video MP4
Download video MP3
Similar videos
-
 0:33
0:33
calculate the molar mass for the following (round atomic mass to the nearest whole number and be su…
-
 0:33
0:33
the molar mass of a substance can be obtained by ________ the atomic masses of the component atoms.
-
 0:33
0:33
how do i calculate calculate the mass, in grams, of 532.0 atoms of cadmium, cd (1 mol of cd has a m…
-
 0:33
0:33
using the following equation for the combustion of octane, calculate the heat associated with the f…
-
 0:33
0:33
calculate the molar mass of each of the following compounds: (a) hydrogen fluoride, hf (b) ammonia,…
-
 0:33
0:33
calculate the mass in grams, 0f 922 atoms has of iron, mass 0f 55.85 9). (1 mol of fe 0@
-
 0:33
0:33
calculate the percentage by mass of oxygen in the following compounds: (a) morphine, c_17…
-
 0:33
0:33
the element lead (pb) consists of four naturally occurring isotopes with atomic masses 203.97302,20…
-
 0:33
0:33
(ii) calculate the maximum mass of ethanol that could be obtained from 30.0g of glucose
-
 0:33
0:33
the molar mass of an atomic element has the same value as the weight of that atom in atomic mass un…
-
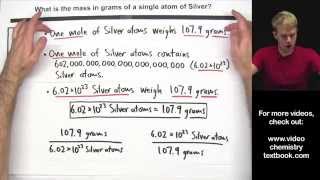 7:16
7:16
calculate the mass of a single atom or molecule
-
 0:33
0:33
calculate the molarity of each of the following solutions. 1. 0.600 mol of bacl2 in 100.0 ml of sol…
-
 0:33
0:33
calculate the atomic mass of the chlorine-37 nucleus in amu (to three decimal places) assuming that…
-
 0:33
0:33
calculate the energy required to decompose 57.0 kg of fe3o4 according to the following reaction: fe…
-
 0:33
0:33
1) calculate the molar mass of nh₃ (include units, round to the nearest whole number) 2) calculat…
-
 0:33
0:33
calculate the number of moles in each of the following masses: a. 3.00 g of boron tribromide, bbr3 …
-
 0:33
0:33
q8: calculate the quantity indicated for each of the following electrolyses. a)the mass of zn depos…
-
 0:33
0:33
what is the molar mass of an unknown gas if 1.60 grams of that gas occupies a volume of 2.24 l at 7…
-
 0:33
0:33
the number of atoms in 5.0 g of aluminum is calculated using the formula: number of atoms = (mass o…
Clip.africa.com - Privacy-policy
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
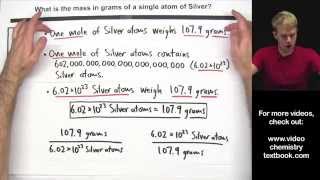 7:16
7:16
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33
 0:33
0:33