calculate the molar solubility of aloh3 , ksp = 2 × 10 −32
Published 3 years ago • 589 plays • Length 6:37Download video MP4
Download video MP3
Similar videos
-
 2:04
2:04
how to calculate molar solubility of al(oh)3
-
 41:52
41:52
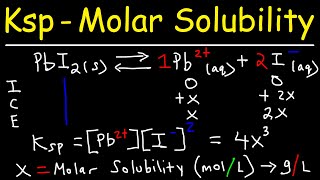
ksp - molar solubility, ice tables, & common ion effect
-
 0:33
0:33
calculate the molar solubility of al(oh) _3, k_88=2 ×10^-32.
-
 10:22
10:22
calculate the molar solubility of calcium hydroxide in a solution buffered at each ph a ph = 4 b
-
 5:45
5:45
⚗️ calculating molar solubility in the presence of a common ion
-
 0:33
0:33
calculate the molar solubility of co(oh)3, ksp = 2.5 x 10-43.
-
 12:28
12:28
calculating the molar solubility of ag2co3 sp 9 a5
-
 11:39
11:39
buffer solution ph calculations | chemistry | khan academy
-
 4:43
4:43
find the ph of a buffer after adding hcl
-
 5:16
5:16
example: determining whether a precipitate will form (solubility equilibrium #3)
-
 9:11
9:11
4.5.3 - calculating molar solubility from ksp
-
 7:11
7:11
calculate the molar solubility of mgoh2, ksp = 89×10-12
-
 3:18
3:18
⚗️ calculating molar solubility from kₛₚ (question 2)
-
 10:27
10:27
37: calculating solubility, molar solubility, and ksp
-
 4:52
4:52
worked example: calculating solubility from kₛₚ | equilibrium | ap chemistry | khan academy
-
 2:55
2:55
chemistry help: calculate the molar solubility cr(oh)3 - chromium (iii) hydroxide
-
 3:26
3:26
⚗️ calculating kₛₚ from molar solubility (ag₂so₄)
-
 4:04
4:04
calculate the molar solubility of aluminum hydroxide
-
 4:45
4:45
molar solubility of pbi2 in 0.10 m nai solution
-
 2:07
2:07
calculate the molar solubility of cd(oh)2 from ksp?
-
 11:41
11:41
15.93 | calculate the molar solubility of al(oh)3 in a buffer solution with 0.100 m nh3 and 0.400 m
-
 5:34
5:34
chem 201: calculating molar solubility of a hydroxide salt in water – common ion effect 3