calculate the molarity of a solution containing 5 g of naoh in a 450 ml solution.
Published 6 months ago • 317 plays • Length 2:37Download video MP4
Download video MP3
Similar videos
-
 5:23
5:23
calculate the molarity of a solution containing 5g of naoh in 450ml solution.
-
 0:18
0:18
calculate the molarity of a solution containing 5g of naoh in 450 ml solution ?
-
 2:46
2:46
calculate the molarity of a solution containing `5 g` of naoh in `450 ml` solution....
-
 2:47
2:47
calculate the molarity of a solution containing `5 g` of naoh in `450 ml` solution.
-
 4:31
4:31
calculate the molarity of a solution containing 5gm of naoh in 450 ml of solution
-
 3:29
3:29
calculate the molarity of a solution containing 5g of naoh in 450ml |class 12 chemistry | doubtnut
-
 2:32
2:32
calculate the molarity of a solution containng 5g of naoh disslced in 450 ml of the solution. | ...
-
 2:36
2:36
calculate the molarity of a solution containing 5g of naoh in 500 ml |class 12 chemistry | doubtnut
-
 3:57
3:57
practice problem: titration calculations
-
 8:46
8:46
molarity made easy: how to calculate molarity and make solutions
-
 21:27
21:27
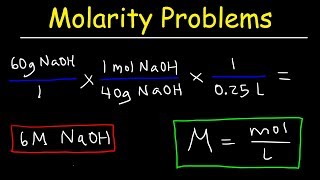
molarity practice problems
-
 6:42
6:42
calculate the molarity of a solution containing 5g of naoh in 450ml of solution | by sisu ojho
-
 31:25
31:25
molarity, molality, volume & mass percent, mole fraction & density - solution concentration problems
-
 4:30
4:30
calculate the molarity of a solution containing 5 g of naoh in 450 ml solution.
-
 2:36
2:36
calculate the molarity of a solution containing 5g of naoh in 500 | class 12 chemistry | doubtnut
-
 4:38
4:38
calculate the molarity of naoh in the solution prepared by dissolving its 4g in enough water .....
-
 2:52
2:52
what is the molarity of a solution prepared by dissolving 3.22 grams of naoh in enough deionized
-
 2:19
2:19
calculate the molarity of a solution containing 1g of naoh in 250 ml |class 12 chemistry | doubtnut
-
 0:26
0:26
prepare 0.1n naoh solution #normality # #shorts #shortsvideo
-
 1:00
1:00
stoichiometry with mass (grams) #chemistry #shorts #education #science #homework
-
 5:07
5:07
calculate the concentration (in molarity) of a naoh solution if 26.0 ml of the solution are needed
-
 7:32
7:32
normality and molarity |0.75 g of nahco3 is dissolved in 250 ml of a solution |solutions chapter