calculate the root mean square (rms) speed of oxygen gas at room temperature
Published 10 years ago • 100K plays • Length 10:00Download video MP4
Download video MP3
Similar videos
-
 2:26
2:26
the root mean square (r.m.s) speed of oxygen molecules `(o_2)` at a certain temperature t (degre...
-
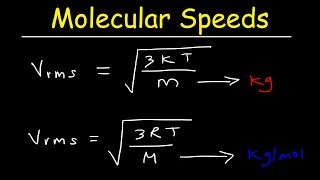 3:54
3:54
molecular speed of gases formula with boltzmann's constant
-
 3:03
3:03
what is the root mean square speed of oxygen molecules at this temperature? (300k)
-
 1:54
1:54
the root mean square (rms) speed of oxygen molecules \( \left(\mathrm{o}_{2}\right) \) at a cert...
-
 12:29
12:29
root mean square velocity - equation / formula
-
 1:39
1:39
the root mean square (rms) speed of oxygen molecules (o_2) at a certain temperature t (degree abs...
-
 2:00:12
2:00:12
gas law problems combined & ideal - density, molar mass, mole fraction, partial pressure, effusion
-
 4:13
4:13
molecular kinetic theory (simple derivation) - kinetic theory (lesson 4)
-
 10:46
10:46
physics 32 kinetic theory of a gas (7 of 10) the maxwell boltzmann distribution
-
 0:32
0:32
calculate rms velocity of oxygen molecule at 27°c(in ms-1)
-
 13:13
13:13
what is root mean square velocity (rms speed)? #6
-
 3:22
3:22
the temperature at which oxygen molecules have the same root mean square speed as that of hydroge...
-
 4:15
4:15
the root mean square (rms) speed of oxygen \( \mathrm{p} \) molecul...
-
 4:15
4:15
the root mean square (rms) speed of oxygen \( \mathrm{p} \) molecul...
-
 4:15
4:15
the root mean square (rms) speed of oxygen \( \mathrm{p} \) molecul...
-
 2:05
2:05
calculate the temperature at which root mean square velocity of \( ...
-
 1:48
1:48
hcv: the rms speed of oxygen at room temperature is about 500 m/s. the rms speed of hydrogen at the
-
 1:47
1:47
find `rms` speed of oxygen molecules at temperature `27^(@)c`.
-
 3:10
3:10
find the temperature t, at which the r.m.s. speed of oxygen molecules equals to escape velocity ...
-
 3:32
3:32
⚗️ root mean square velocity (question 1)
-
 3:44
3:44
the root mean square (rms) speed of oxygen molecules at certain temperature t (absolute) is v, if
-
 0:33
0:33
calculate the rms speed of an oxygen molecule at 8 â°c. determine how many times per second each mo…