how many milliliters of 0.250 m hno _3 are needed to neutralize the following solutions? a. 25.0 …
Published 2 weeks ago • 3 plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 5:48
5:48
unit 5.2: mills methods
-
 0:33
0:33
a chemist mixes 175 milliliters of a solution that is 62
-
 0:33
0:33
a solution contains 1.0 ×10^-5 mol of kbr and 0.10 mol of kcl per liter. agno _3…
-
 0:33
0:33
how many moles of ions are contained in 1 l of a 1 m solution of kcl ? o…
-
 0:33
0:33
calculate the mole fraction of kcl in an aqueous solution which is 1.20 molal in kcl.
-
 8:48
8:48
determining amount of solution for a desired strength (q1c1 = q2c2)
-
 0:33
0:33
use the following electrode potential diagram for basic solutions to classify each of the statement…
-
 0:33
0:33
in the lab, we calculated the coefficient of kinetic friction between a wooden block and our alumin…
-
 9:29
9:29
equilibrium equations: crash course chemistry #29
-
 17:25
17:25
percentage concentration calculations
-
 3:41
3:41
meq & valence
-
 10:11
10:11
find the amount of diluent needed when preparing a solution of lower strength
-
 51:08
51:08
live classroom - review problems for millimoles, milliequivalents, & milliosmoles
-
 1:00:05
1:00:05
electrolyte solutions part 03 - review problems
-
 0:33
0:33
5. ppm (parts per million) is a term used in chemistry to denote a very low concentration of a solu…
-
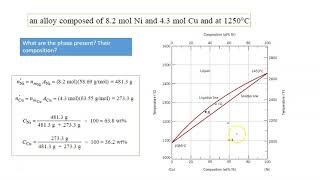 10:17
10:17
phase diagram (materials science) - part 3
-
 6:49
6:49
how to solve milliequivalents calculations example 1
-
 10:29
10:29
calculating the number of moles at equilibrium from kc
-
 0:33
0:33
a. calculate the ph of a buffered solution that is 0.100 m in c_6 h_5 …
-
 7:40
7:40
calculating cell potential at non-standard conditions (nernst equation)
-
 10:05
10:05
07f an example with module factors
-
 1:55
1:55
how biomedical engineering students can determine phase fraction using lever rule in a phase diagram