camphor is soluble in water. the dominant intermolecular force(s) present between the solute and so…
Published 9 months ago • 9 plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 4:29
4:29
how solubility and dissolving work
-
 4:23
4:23
solubility and intermolecular forces | ap chemistry | khan academy
-
 45:36
45:36
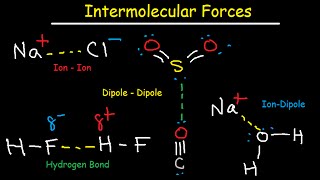
intermolecular forces - hydrogen bonding, dipole-dipole, ion-dipole, london dispersion interactions
-
 10:54
10:54
intermolecular forces and boiling points
-
 5:19
5:19
what are intermolecular forces | properties of matter | chemistry | fuseschool
-
 3:52
3:52
how polarity makes water behave strangely - christina kleinberg
-
 0:33
0:33
what kinds of intermolecular forces are present in a mixture of potassium chloride and water?
-
 10:54
10:54
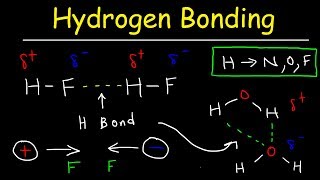
hydrogen bonds in water explained - intermolecular forces
-
 5:03
5:03
why don't oil and water mix? - john pollard
-
 8:07
8:07
intermolecular forces | chemistry
-
 10:40
10:40
intermolecular forces - hydrogen bonding, dipole dipole interactions - boiling point & solubility
-
 2:01
2:01
intermolecular forces for h2o (water)
-
 12:29
12:29
intermolecular forces - ion, dipole, london dispersion and hydrogen bonds explained
-
 6:57
6:57
imf and solubility
-
 5:10
5:10
intermolecular forces magic trick
-
 6:39
6:39
hydrogen bonding | intermolecular forces and properties | ap chemistry | khan academy
-
 1:00
1:00
polar vs nonpolar - amoeba sisters #shorts
-
 4:58
4:58
s2.2.8 solubility and intermolecular forces
-
 5:52
5:52
intermolecular forces experiment