calculate the solubility of agcn(s)(k_sp=2.2 ×10^-12) …
Published 4 days ago • No plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 1:57
1:57
how to find the solubility product constant (ksp) | profound brains
-
 4:52
4:52
worked example: calculating solubility from kₛₚ | equilibrium | ap chemistry | khan academy
-
 8:36
8:36
solubility product constant (ksp)
-
 14:31
14:31
chem203: experiment 8: solubility and solubility product
-
 4:29
4:29
how solubility and dissolving work
-
 41:52
41:52
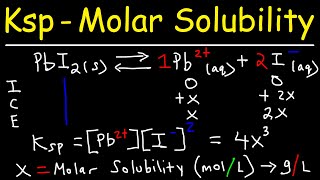
ksp - molar solubility, ice tables, & common ion effect
-
 16:42
16:42
determination of solubility product of sparingly soluble salt // b.sc // jam // net // gate // set
-
 2:55
2:55
how to calculate solubility from solubility product
-
 1:31
1:31
understanding the solubility product constant
-
 0:33
0:33
write the expression for the solubility product constant of mgf_2( see problem …
-
 3:55
3:55
lesson 8: solubility product (ksp) of ag2cro4 | mathematical problem | topic: solubility
-
 6:09
6:09
slightly soluble 8.1 - ksp
-
 0:33
0:33
find molar solubility and ksp, based on the number of potential cations and anions in ag2so4 and sr…
-
 7:36
7:36
aleks: writing a solubility product (ksp) expression
-
 3:25
3:25
how to calculate the solubility of a sparingly soluble salt
-
 1:48
1:48
for the electrolyte of type, `a_(2)b, k_(sp)` is given then its solubility is calculated by
-
 1:10
1:10
solubility product (ksp) sample problem: chapter 17 – part 11
-
 2:35
2:35
the solubility product `(k_(sp))` of the sparingly soluble salt `mx` at `25^(@)c` is `2.5xx10^(-9)`.
-
 0:33
0:33
saccharin, a sugar substitute, is a weak acid with pk_a=2.32 at 25^∘ c . …