consider the equation you react 4 moles of a with 2 moles of which of the following is true a the
Published 1 month ago • 9 plays • Length 3:52Download video MP4
Download video MP3
Similar videos
-
 12:11
12:11
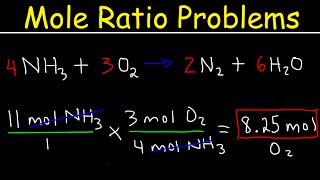
stoichiometry mole to mole conversions - molar ratio practice problems
-
 0:46
0:46
the number of moles of co produced when 3 moles of hcl react with excess of caco3
-
 25:16
25:16
stoichiometry basic introduction, mole to mole, grams to grams, mole ratio practice problems
-
 5:13
5:13
the balanced equation for a reaction is given below 2x 3y → 4l m when 8 moles of x react with 15
-
 5:16
5:16
introduction to moles
-
 27:15
27:15
the most misunderstood concept in physics
-
 7:29
7:29
the big idea behind avogadro's number (that most people miss)
-
 1:32:12
1:32:12
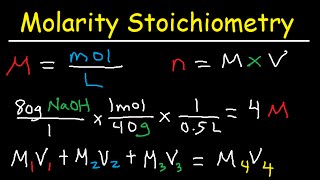
molarity dilution problems solution stoichiometry grams, moles, liters volume calculations chemistry
-
 8:44
8:44
how to find the mole ratio to solve stoichiometry problems
-
 8:00
8:00
a)how many moles of o2 are needed to react fully with 4 moles of octane? to be continued
-
 1:01:57
1:01:57
stoichiometry with limiting reactant
-
 7:02
7:02
mole-to-mole conversions
-
 4:12
4:12
which if any of the following isare true regarding the limiting reactant in a chemical reaction a t
-
 4:29
4:29
gcse chemistry - the mole (higher tier) #25
-
 0:56
0:56
the number of moles of co{2} produced when 3 moles of hci react with excess of caco
-
 0:45
0:45
the number of moles of c*o_{2} produced when 5 moles of hcl react with excess of caco 3 is
-
 0:33
0:33
write the balanced chemical equation for a reaction where 4 moles of ammonia and 5 moles of oxygen …
-
 5:54
5:54
calculating masses in reactions - p27 (chem)
-
 0:39
0:39
the number of moles of c*o_{2} produced when 5 moles of hcl react with excess of caco 3 is
-
 13:10
13:10
the mole map
-
 13:37
13:37
an actually good explanation of moles
-
 0:30
0:30
asking minor test marks to allen topper allen kota #allen #allenkota #physicswallah #pw