cp-cv =r | difference of two molar specific heat is equal to universal gas constant | heat | physics
Published 3 years ago • 96 plays • Length 32:41Download video MP4
Download video MP3
Similar videos
-
 11:32
11:32
thermodynaimcs 07 || derivation of cp - cv = r , mayer's relation important for school exams ||
-
 12:39
12:39
thermodynamics specific heats - cv & cp - in 12 minutes!
-
 4:14
4:14
heat capacity, specific heat, and calorimetry
-
 11:13
11:13
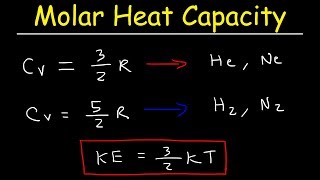
molar heat capacities of gases, equipartition of energy & degrees of freedom
-
 0:17
0:17
cp-cv=r proof | physics | #shorts #thermodynamics #physics #heat #derivation #neet #jee
-
 32:45
32:45
molar specific heat of gas class 11 | derive cp - cv = r | relation between cp and cv
-
 12:34
12:34
prove cp - cv = r ; where cp & cv are the molar specific heat of a gas at constant pressure & volume
-
 8:35
8:35
7.4 specific heat difference cp-cv of an ideal gas
-
 4:00
4:00
physics made easy- specific heat of gas - cp cv derivation
-
 3:22
3:22
09 relation between two molar specific of gas (cp & cv)
-
 7:09
7:09
cp-cv=r, relation between specific heats, cp=cv r, gtu paper solution, #mechanical #imp
-
 10:47
10:47
molar specific heats of a gas relation between them| cp is greater than cv || thermodynamics
-
 3:39
3:39
proof | meyer's equation | cp-cv=r
-
 11:18
11:18
molar specific heat for constant volume and constant pressure
-
 6:07
6:07
cp - cv=r; another way
-
 10:26
10:26
university physics lectures, molar specific heat of an ideal gas
-
 7:20
7:20
inter i yr : physics i (e/m) topic : thermodynamics part 7 prove that (cp-cv=r)
-
 3:08
3:08
cv and cp
-
 11:58
11:58
relationship between cp and cv | cp - cv = r | specific heat at constant pressure and volume