demystifying the process of approaching the fda as a medtech innovator
Published 2 years ago • 126 plays • Length 53:51Download video MP4
Download video MP3
Similar videos
-
 49:36
49:36
catalyze seminar: gaining the edge - medical device products development to regulatory
-
 1:57
1:57
how the fda classifies medical devices | by proxima cro
-
 6:11
6:11
innovation pathway at fda
-
 4:23
4:23
navigating the fda medical device classification process
-
 49:08
49:08
medical devices 101: an entry level overview of the fda
-
 42:51
42:51
where do the acceptance criteria in method validation come from? - webinar recording
-
 1:34:30
1:34:30
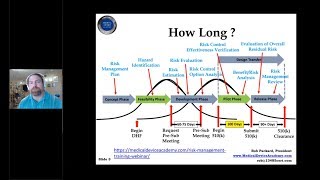
how to prepare a medical device 510k submission for fda | rob packard | joe hage
-
 31:54
31:54
webinar: medical devices software development and applications
-
 1:11:29
1:11:29
2019 mti live webinar 4: regulatory strategy for medtech startups
-
 0:14
0:14
highest paid healthcare workers 💰 (that aren't medical doctors) #shorts
-
 6:05
6:05
fda drug approval process trailer
-
 0:32
0:32
can everyone say, “acceptance criteria”? #510(k) #fda #validation
-
 33:59
33:59
128 - look for the helpers: interviews with medc and medtech innovator
-
 5:47
5:47
fda medical device approval process trailer
-
 1:05:36
1:05:36
2020 mti apac webinar 2: medtech m&a & working with strategics
-
 1:49
1:49
navigating the fda 513(g) process - an essential guide
-
 2:55
2:55
what fda data tells us about medtech innovation
-
 54:13
54:13
114 - discover strategies for de-risking medical device development: fda consensus standards
-
 1:02:13
1:02:13
medtech innovation basics: regulatory plan & quality management systems - medventions lecture series
-
 0:37
0:37
know your user #medicaldevices #fda
-
 3:40
3:40
what advice can you offer first-time medtech innovators?