determine the standard cell potential for a voltaic cell made of a ag strip and an al strip immerse…
Published 6 months ago • 1 view plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 0:33
0:33
consider the reaction corresponding to a voltaic cell and its standard cell potential. z n ( s ) …
-
 0:33
0:33
a voltaic cell that uses the following reaction has a measured standard cell potential of 1.03 v. …
-
 0:33
0:33
write the balanced equation of the reaction that occurs in a voltaic cell made using aluminum and t…
-
 0:33
0:33
a voltaic cell similar to that shown in figure 20.5 in the textbook is constructed. one electrode c…
-
![a voltaic cell designed to measure [cu^2] is constructed of a standard hydrog…](https://i.ytimg.com/vi/9kjWyHKZkao/mqdefault.jpg) 0:33
0:33
a voltaic cell designed to measure [cu^2] is constructed of a standard hydrog…
-
 11:27
11:27
the nernst equation | applications of thermodynamics | ap chemistry | khan academy
-
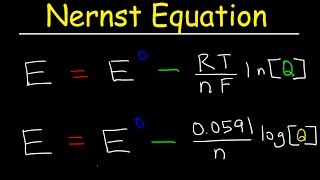 30:53
30:53
nernst equation explained, electrochemistry, example problems, ph, chemistry, galvanic cell
-
 7:40
7:40
calculating cell potential at non-standard conditions (nernst equation)
-
![a voltaic cell is constructed that uses the following half-cell reactions: [ … ]](https://i.ytimg.com/vi/MYGnTEBjyK4/mqdefault.jpg) 0:33
0:33
a voltaic cell is constructed that uses the following half-cell reactions: [ … ]
-
 0:33
0:33
apply suppose that you have a voltaic cell in which one half-cell is made up of a strip of tin imme…
-
 4:54
4:54
how to find the cell potential (ecell) under standard conditions
-
 0:33
0:33
select all that are true for both a voltaic cell and an electrolytic cell the electrodes will chang…
-
 0:33
0:33
a voltaic cell is constructed with an ag/ag ^ half-cell and a pb/pb ^2 half-cell. the silver …
-
 0:33
0:33
a voltaic cell is constructed from an ni^2 (a q)-ni(s) half-cell and an …
-
 0:33
0:33
a voltaic cell is based on the reduction of ag^ (a q) to ag(s) and the oxidatio…
-
 6:59
6:59
standard cell potential | applications of thermodynamics | ap chemistry | khan academy
-
 0:33
0:33
a voltaic cell consists of a zn / zn^2 half-cell and a ni / n…
-
 0:33
0:33
a voltaic cell is constructed with two hydrogen electrodes: electrode 1 has p(h2) = 1.00 atm and an…
-
 0:33
0:33
the voltaic cell represented by: zn(s) | zn2 (aq, 0.100 m) ‖ cl2(g, 0.500 m) | cl- (? m) | pt(s) …
-
 0:33
0:33
a voltaic cell consists of a cd/cd2 electrode (eâ° = -0.40 v) and a fe/fe2 electrode (eâ° = -0.44…
-
 0:33
0:33
urgent plz find the cell potential for each of the below reactions: a. cu(s) znso4(aq) – zn(s) …
-
 0:33
0:33
in a voltaic cell similar to the zn / cu^2 cell in figure 17.2 the cu electrod…