elect. ex. 3- non standard reduction electrode potential (q.4,6,5)
Published 4 years ago • 209 plays • Length 24:14Download video MP4
Download video MP3
Similar videos
-
 10:56
10:56
cell potential problems - electrochemistry
-
 30:53
30:53
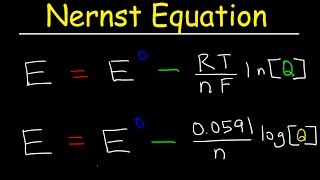
nernst equation explained, electrochemistry, example problems, ph, chemistry, galvanic cell
-
 11:02
11:02
cell potential & gibbs free energy, standard reduction potentials, electrochemistry problems
-
 6:21
6:21
electrochemistry
-
 11:48
11:48
standard reduction potentials of half reactions - electrochemistry
-
 11:27
11:27
the nernst equation | applications of thermodynamics | ap chemistry | khan academy
-
 4:10
4:10
voltaic cell | how does it work?
-
 4:42
4:42
electrochemistry | the standard reduction potential.
-
 9:10
9:10
standard reduction potentials | redox reactions and electrochemistry | chemistry | khan academy
-
 9:04
9:04
electrochemistry: crash course chemistry #36
-
 0:19
0:19
standard electrode potential value | chemistry
-
 9:11
9:11
lec20 - electrode potential and non-standard conditions
-
 1:27:17
1:27:17
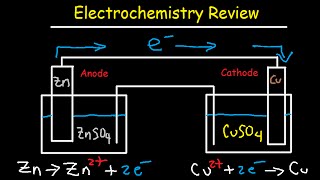
electrochemistry review - cell potential & notation, redox half reactions, nernst equation
-
 7:40
7:40
calculating cell potential at non-standard conditions (nernst equation)
-
 21:45
21:45
measuring standard electrode potentials (electrochemistry #6)
-
 4:55
4:55
standard electrode potentials 3. chemistry and conventions
-
 0:30
0:30
did you know how to remember reactivity series?
-
 36:40
36:40
video 5: factors affecting electrode potential ( electrochemistry)
-
 29:35
29:35
elect. ex. 5b -electrochemical cells
-
 37:45
37:45
electrode potentials: non standard condition (nerst equation)
-
 0:42
0:42
electrochemistry- standard reduction potential