enhancing fda inspections: insights from cyntegrity experts
Published 1 year ago • 101 plays • Length 2:11Download video MP4
Download video MP3
Similar videos
-
 3:10
3:10
introducing cyntegrity and the myrbqm portal
-
 2:36
2:36
cyntegrity 2022 what a year! a message from our ceo...
-
 0:50
0:50
myrbqm fsp - centralized monitoring part 1
-
 58:37
58:37
2023 rbm fda guidance | mindson rbqm
-
 59:31
59:31
how to prepare for your next fda inspection
-
 18:18
18:18
risk-based monitoring - risk-optimized approaches to clinical trials - introduction - part 1 of 3
-
 1:08:18
1:08:18
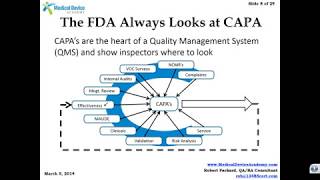
how fda trains its investigators to review capa and what you should do to prepare
-
 2:18
2:18
stratification in a study | myrbqm experts
-
 1:26:58
1:26:58
current fda and ema inspection findings: lessons learned trailer
-
 57:50
57:50
fda good clinical practice inspection trends
-
 1:49
1:49
cyntegrity: risk-based quality management
-
 5:53
5:53
tmf/etmf regulatory agency expectations, inspections, and findings
-
 2:34
2:34
how to deal with unexpected clinical study occurrences?
-
 0:40
0:40
phase ii vanguards - clinical research data visualizations
-
 2:59
2:59
fda denies mdma for ptsd treatment: what’s next for psychedelic therapy?
-
 34:02
34:02
preparing for fda remote inspections
-
 0:47
0:47
changing roles in clinical trial conduct
-
 40:36
40:36
fda's clinical trial inspections in china
-
 1:44
1:44
preparing clinical research sites for fda inspection trailer
-
 1:47
1:47
d-i-g-r: root cause analysis approach in clinical research (d)
-
 2:08
2:08
what makes an auditable kri in clinical research