fill in the orbital energy diagram for the neon atom: 2p e 25 1s submit answer try another version …
Published 10 months ago • 1 view plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 0:33
0:33
fill in the orbital energy diagram for the fluoride ion [55 2p 5 e 25 is
-
 9:52
9:52
filling the orbital energy diagram
-
 11:05
11:05
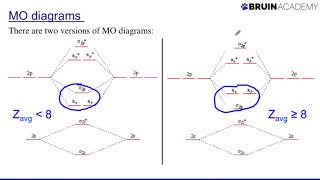
drawing molecular orbital diagrams
-
 0:33
0:33
in the molecule sf4, sulfur makes four covalent bonds. therefore, four of its six valence electrons…
-
 22:49
22:49
how to draw an energy level diagram of an atom in chemistry
-
 0:33
0:33
which orbital in each of the following pairs is lower in energy? a. 2s, 2p b. 3p, 2p c. 3s, 4s
-
 0:33
0:33
the electron configuration of a neutral atom is 1 s^2 2 s^2 2 p^6 3 s^2 . write a complete …
-
 0:33
0:33
the orbital diagram for a ground-state nitrogen atom is 1s a_ 4 _l b 1 _1_ cf 1 lll d 1 1 1ll
-
 0:33
0:33
what is the notation for the subshell in which n=4 and l=3 ? how many orbitals are in this subshell?
-
 0:33
0:33
please draw an energy diagram for the endothermic reaction of nitrogen and oxygen to form nitric ox…
-
 0:33
0:33
consider an electron in a hydrogen atom that moves from bohr orbit n = 3 to n = 2. a.what is the wa…
-
 3:02
3:02
chemistry - electron structures in atoms (40 of 40) orbital energy diagram
-
 0:33
0:33
which of the following orbital diagrams represents a paramagnetic atom? 1s 2s 2p
-
 0:33
0:33
the electron in a hydrogen atom makes transition from the orbital pictured on the left to the orbit…
-
 0:33
0:33
a large number of neon atoms are in thermal equilibrium. what is the ratio of the number of atoms i…
-
 0:33
0:33
n is known as the fill in the blank 1 quantum number. ... l is known as the fill in the blank 2 qua…
-
 0:33
0:33
(a) if an atom has an electron in the n = 7 shell with magnetic quantum number ml = 5, what are the…
-
 0:33
0:33
molecular orbital theory predicts that two ne atoms should not form a covalent bond because: there …
-
 0:33
0:33
which orbital notation represents an atom in the ground state with 6 valence electrons? a)