how many ml hcl of a 0.100 m hcl solution is needed to completely neutralize 25.0 ml of a 0.350 m n…
Published 4 weeks ago • 4 plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 5:33
5:33
how many ml of 0.160 m hclo4 solution are needed to neutralize 33.5 ml of 0.0880 m naoh?
-
 0:33
0:33
calculate the volume of 0.100 m hcl solution needed to neutralize 25.0 ml of 0.350 m naoh solution.…
-
 4:33
4:33
how many ml of 1m h2so4 is needed to neutralize 10ml of 1m of mg(oh)2?
-
 0:33
0:33
if 18.0 ml of a 0.150 m naoh solution is required to neutralize 25.0 ml of an hcl solution, what is…
-
 1:57
1:57
how much of naoh is required to neutralise 1500 `cm^(3)` of `0.1` m hcl?
-
 0:33
0:33
(a) how many milliliters of 0.120 m hcl are needed to completely neutralize 50.0 m…
-
 4:51
4:51
if 12.5 ml of a 0.144 m naoh solution is required for neutralization of 6.0 ml of given hcl solution
-
 2:05
2:05
oqv no – 285 what volume of 0.4 (m) naoh is needed to neutralize 50 ml of 0.2 (m) hcl?
-
 9:24
9:24
titration of hcl with naoh
-
 8:56
8:56
titration: practical and calculation (naoh and hcl)
-
 1:32:12
1:32:12
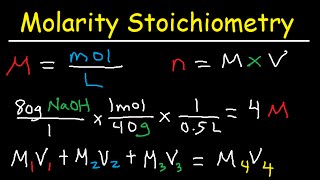
molarity dilution problems solution stoichiometry grams, moles, liters volume calculations chemistry
-
 11:11
11:11
how to prepare 1m hcl solution | preparation of 0.1m hcl solution
-
 11:04
11:04
100. ml of 0.200 m hcl is titrated with 0.250 m naoh. part a 1)what is
-
 0:19
0:19
how much hydrochloric acid is required to neutralize 10 ml of sodium hydroxide?
-
 3:01
3:01
20.0 ml of a 3.0m hcl solution are mixed with 20.0 ml of a 5.0m naoh solution. what is the ph?
-
 5:07
5:07
calculate the concentration (in molarity) of a naoh solution if 26.0 ml of the solution are needed
-
 0:33
0:33
if 10.0 ml of 0.100 m hcl is titrated with 0.200 m naoh, what volume of sodium hydroxide solution i…
-
 0:33
0:33
0.200 m sodium hydroxide (naoh) being added to 30 ml of hydrochloric acid (hcl) of unknown concentr…
-
 1:37
1:37
find the ph of a 0.1m hcl (hydrochloric acid) solution
-
 0:33
0:33
assigned as homework 0 q16.39 a 10.0 ml sample of an hcl solution is titrated with 1.00 m naoh solu…
-
 0:41
0:41
experiment to show #turmeric (#haldi ) as a natural #indicator..! #red #colour in #detergent (base)
-
 0:58
0:58
neutralization reaction of hydrochloric acid and sodium hydroxide