how many orbitals are there in an atom with the following combinations of quantum numbers? a. n=3, …
Published 7 days ago • No plays • Length 0:33
Download video MP4
Download video MP3
Similar videos
-
 3:46
3:46
how many orbitals are there in n=3, the 3rd energy level of an atom?
-
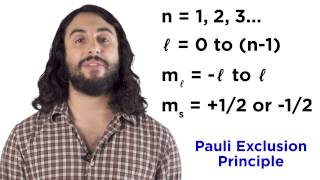 8:42
8:42
quantum numbers, atomic orbitals, and electron configurations
-
 11:46
11:46
how to determine the maximum number of electrons using allowed quantum numbers - 8 cases
-
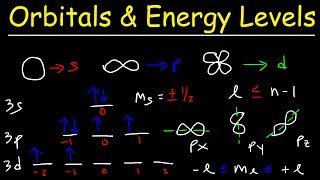 11:19
11:19
orbitals, atomic energy levels, & sublevels explained - basic introduction to quantum numbers
-
 12:16
12:16
quantum numbers
-
 2:13:38
2:13:38
lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar
-
 21:34
21:34
what are atomic orbitals?
-
 5:50
5:50
atomic orbitals 3d
-
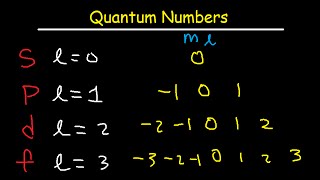 4:25
4:25
how to determine the 4 quantum numbers from an element or a valence electron
-
 12:01
12:01
spdf orbitals explained - 4 quantum numbers, electron configuration, & orbital diagrams
-
 7:20
7:20
quantum numbers - how many electrons and orbitals have the following set of quantum numbers?
-
 4:34
4:34
college chemistry: label each of the following sets of quantum numbers as a valid or invalid
-
 7:00
7:00
advanced higher chemistry - atomic orbitals & quantum numbers
-
 9:23
9:23
shells, subshells, and orbitals - biology/chemistry ep5
-
 5:56
5:56
atomic orbitals simply explained! inorganic chem - 1.12
-
 2:07
2:07
how many quantum number are needed in designate an orbital ? name them
-
 5:05
5:05
explain giving reasons which of the following sets of quantum numbers are possible a) n=0,l=0,ml=0,
-
 0:26
0:26
finding protons, electron, neutrons | chemistry class 9 / 10 science | youtube shorts by jp sir
-
 32:31
32:31
chemistry 20ap - quantum numbers - atomic orbitals (s,p,d,f)
-
 0:34
0:34
how many electrons in an atom may have the quantum number n=3 , l=0 //q13
-
 0:48
0:48
how small are atoms?
-
 38:44
38:44
orbitals, quantum numbers & electron configuration - multiple choice practice problems
Clip.africa.com - Privacy-policy
 3:46
3:46
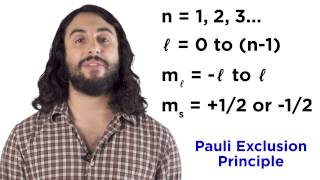 8:42
8:42
 11:46
11:46
 11:19
11:19
 12:16
12:16
 2:13:38
2:13:38
 21:34
21:34
 5:50
5:50
 4:25
4:25
 12:01
12:01
 7:20
7:20
 4:34
4:34
 7:00
7:00
 9:23
9:23
 5:56
5:56
 2:07
2:07
 5:05
5:05
 0:26
0:26
 32:31
32:31
 0:34
0:34
 0:48
0:48
 38:44
38:44