how to handle u.s. fda detentions and import alerts
Published 6 years ago • 1.6K plays • Length 48:35Download video MP4
Download video MP3
Similar videos
-
 47:45
47:45
u.s. fda drug detentions: causes, consequences, and tools to assist
-
 0:48
0:48
fast fda faq - what is an fda import alert?
-
 59:19
59:19
how to prepare for an fda inspection
-
 6:42
6:42
import alert | examples and categories
-
 47:21
47:21
fast facts on fsma: fda voluntary qualified importer program (vqip)
-
 6:59
6:59
clever way to make indestructible boxes, cabinets and drawers
-
 7:20
7:20
how to wire fire control module in (fdas)
-
 11:33
11:33
how yama seafood sells 8,000 pounds of tuna to nyc's michelin-starred restaurants — vendors
-
 1:03
1:03
bite lens: fda import alerts
-
 10:55
10:55
importing fda-regulated products: the import process
-
 1:01:22
1:01:22
six factors that affect your shipments to the u.s.
-
 19:48
19:48
filing prior notice with fda
-
 49:06
49:06
3 ways to protect your brand with facility 360
-
 3:06
3:06
detention & refusal of imported products
-
 16:02
16:02
importing fda-regulated products: seafood
-
 3:16
3:16
facility 360 by registrar corp - monitor your shipments and protect your brand
-
 1:01:20
1:01:20
fda registration and u.s. labeling requirements for winemakers
-
 1:01:23
1:01:23
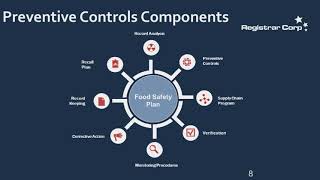
u.s. fda preventive controls requirements
-
 1:35:08
1:35:08
webinar: us fda imports overview
-
 1:34:58
1:34:58
practical steps to be mocra compliant quickly