14.16 | the ionization constant for water (kw) is 2.9 × 10^−14 at 40 °c. calculate [h3o ], [oh−], ph
Published 2 years ago • 6.2K plays • Length 9:13Download video MP4
Download video MP3
Similar videos
-
 24:59
24:59
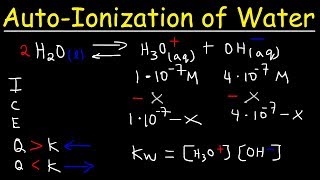
autoionization of water, ion product constant - kw, calculating h3o , oh-, and ph using ice tables
-
 5:03
5:03
r3.1.5 ionic product constant of water, kw
-
 5:18
5:18
ibdp chemistry hl: kw at different temperatures
-
 6:01
6:01
what is kw (the ion product constant of water)
-
 5:16
5:16
the kw for water at 0 °c is 0 12× 10–14 calculate the ph of a neutral aqueous solution at 0 °c
-
 6:35
6:35
the kw for water at 0°c is 012× 10–14 calculate the ph of a neutral aqueous solution at 0 °c
-
 16:20
16:20
kw, ionic product of water (a-level chemistry)
-
 25:57
25:57
baiki/repair water heater ep.43: deka e800, no power, air tak panas dan pump juga tidak berfungsi.
-
 5:02
5:02
unboxing panasonic a-130jack water pump shallow well (bahasa malaysia) | atkc hardware
-
 3:11
3:11
calculate the ph of water at 25°c and 75°c.
-
 33:21
33:21
buffer solutions
-
 6:49
6:49
kac27.11 - acids & ph: ph calculations - pure water
-
 4:21
4:21
8.3 ionic product constant of water kw (sl)
-
 5:06
5:06
⚗️ using kw in calculations
-
![14.17 | the ionization constant for water (kw) is 9.311 × 10^−14 at 60 °c. calculate [h3o ], [oh−]](https://i.ytimg.com/vi/P4hbM8-esQY/mqdefault.jpg) 8:02
8:02
14.17 | the ionization constant for water (kw) is 9.311 × 10^−14 at 60 °c. calculate [h3o ], [oh−]
-
 1:56
1:56
calculate the ph of water at 100c if ionic product of water is 55 times than that at 25c
-
 9:05
9:05
deriving the value of kw: proving kw = 1.0x10^-14 | grade 10 chemistry | lecture 14
-
 10:35
10:35
ph and poh | ionic product of water | chemistry
-
 12:54
12:54
1032 dissociation of water and kw
-
 10:54
10:54
how to calculate ph of water
-
 5:46
5:46
the autoionization of water and kw (pt 2)
-
 4:14
4:14
solving for the ph of pure water at different temperatures