list of mandatory documents for iso 13485 & fda 21 cfr 820 compliance
Published 1 year ago • 74 plays • Length 2:37Download video MP4
Download video MP3
Similar videos
-
 19:07
19:07
fda's transition from cfr 820 to the iso 13485:2016 instituting a new qms
-
 12:48
12:48
why does 21 cfr 820 need to be modernized to iso 13485?
-
 11:47
11:47
revolutionize compliance: shifting from fda 21 cfr part 820 to iso 13485
-
 0:16
0:16
what even are iso 13485 and 21 cfr 820? #fda #iso13485 #21cfr #medicaldevice
-
 3:36
3:36
document control 820.40 & iso 13485 § 4.2.1 & 4.2.4 (executive series #22)
-
 7:13
7:13
what is 21 cfr 820?
-
 1:34
1:34
what is iso 13485 certification? what is 21 cfr 820 compliance?
-
 48:46
48:46
grandmaster #11 - navigating us regulatory pathways for medical devices and samd by amit gururajan
-
 1:15:28
1:15:28
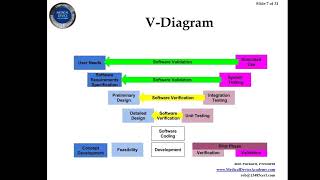
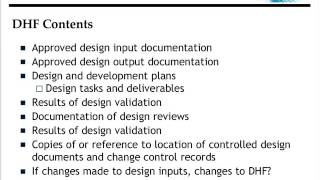
how to you create a design history file (dhf)?
-
 45:34
45:34
qmsr masterclass - everything you need to know
-
 0:36
0:36
fda 21 cfr part 820 quality system regulation
-
 5:15
5:15
gmp for medical devices overview ( fda 21 cfr 820 )
-
 2:39
2:39
iso 13485 & fda cfr 21 part 820 quality management systems - medical devices
-
 0:46
0:46
top 5 benefits of 21 cfr part 820 - quality system regulations for medical devices
-
 24:20
24:20
overview of the quality system regulation
-
 1:02:57
1:02:57
fda quality systems regulation requirements - regulatory documents explained
-
 3:25
3:25
united states medical device registration chapter 3 - quality management system
-
 3:31
3:31
records 820.180 & iso 13485 § 4.2.5 (executive series #23)
-
 3:29
3:29
quality system 21 cfr 820.5 & iso 13485 § 4.1.1 – 4.1.4 (executive series #57)
-
 1:21
1:21
fda updated qsr – 21 cfr, part 820 information
-
 18:12
18:12
iso 13485 overview and section 4