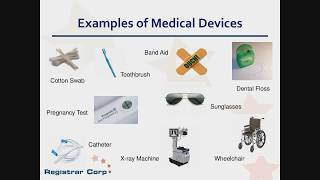
medical device 101 - class i, ii, iii & de novo explained
Published 2 years ago • 1.2K plays • Length 3:50Download video MP4
Download video MP3
Similar videos
-
 4:58:23
4:58:23
webinar: introduction to us fda medical device regulations (510k, de novo, ide, capa, emdr)
-
 49:08
49:08
medical devices 101: an entry level overview of the fda
-
 9:28
9:28
medical device regulations / fda approval
-
 16:18
16:18
how is my medical device classified?
-
 4:23
4:23
navigating the fda medical device classification process
-
 57:47
57:47
fda 101 for medical devices
-
 1:00
1:00
justifying an fda device classification and the regulation name and number for de novo (july 11)
-
 29:33
29:33
episode 23: de novo classification process (evaluation of automatic class iii designation)
-
 54:15
54:15
medical device regulation (fda)
-
 15:35
15:35
what is a de novo?
-
 0:52
0:52
understanding fda classifications: navigating medical device safety requirements
-
 3:02
3:02
medical device 102 - classification of medical devices
-
 4:11
4:11
what is the difference between a 510k and de novo?
-
 0:16
0:16
medical device classification/fda regulations
-
 10:59
10:59
medical devices classification as per fda | medical device regulations | #medicaldevices #fda
-
 2:29
2:29
medical device registration in the us- the de novo request application process
-
 18:53
18:53
what is the regulatory pathway for a de novo medical device or ivd?
-
 1:17:01
1:17:01
fda overview and tips on identifying predicate devices
-
 0:51
0:51
prepare your medical devices for the latest fda cybersecurity guidelines