method development and validation for the estimation of bortezomib in pure and its pharmaceutical
Published 1 year ago • 27 plays • Length 3:23Download video MP4
Download video MP3
Similar videos
-
 26:05
26:05
the finalized bioanalytical method validation guidance: what’s new for ndas and blas – june 17, 2019
-
 4:33
4:33
development of an analytical method and validation of exemestane tablet by uv spectrophotometry
-
 5:53
5:53
development and validation of hplc assay method of tofisopam by qbd approach
-
 13:54
13:54
quality assistance - development and validation of an antibody-drug conjugate bioassay
-
 31:11
31:11
webinar: assay development – from scratch to validated assays
-
 24:30
24:30
bioanalytical method validation: history, process, and regulatory perspectives - bioanalysis 2020
-
 16:11
16:11
all about velcade (bortezomib) for myeloma patients #myeloma
-
 37:20
37:20
webinar: challenges & solutions development and validation of abatacept biosimilar clinical assays
-
 49:01
49:01
overcome the challenges of pk assay development using trailblazer antibodies
-
 2:00
2:00
combining bortezomib and lenalidomide-based regimens for frontline mm
-
 3:52
3:52
choosing validated antibodies for trustworthy data
-
 25:20
25:20
precision dosing of antibody therapeutics
-
 3:19
3:19
western blotting without trial and error: use precisionab™ antibodies
-
 40:31
40:31
applications of metagenomic ngs in immunodeficient patients
-
 53:53
53:53
lessons from 20 years in antibody validation and reproducibility
-
 7:51
7:51
why are 'bio' test methods so tricky to validate?
-
 10:17
10:17
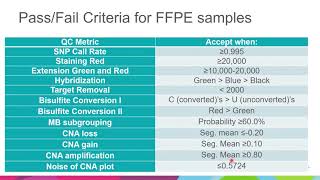
expansion of the validation of illumina methylationepic beadchip for medulloblastoma subgrouping a
-
 54:21
54:21
overview of the use of blood transfusions in different clinical settings