new fda draft guidance for chemical analysis
Published 13 days ago • 22 plays • Length 13:54Download video MP4
Download video MP3
Similar videos
-
 39:15
39:15
cgmps for combination products: understanding and applying fda’s draft guidance - barr weiner of fda
-
 6:05
6:05
fda inspection and compliance : regulatory requirements and best practices
-
 7:00
7:00
breaking down fda's insane draft guidance on choosing a predicate
-
 1:00:28
1:00:28
writing the “indications and usage” section of labeling: fda’s new draft guidance – sep. 27, 2018
-
 41:15
41:15
understanding the new fda guidance on data standards
-
 48:41
48:41
computer software assurance (csa): understanding the fda’s new draft guidance
-
 1:39:35
1:39:35
inspection of injectable products for visible particulates fda guidance
-
 1:51:49
1:51:49
auditing analytical laboratories for fda compliance
-
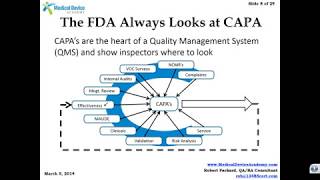 59:31
59:31
how to prepare for your next fda inspection
-
 0:44
0:44
how do you create a risk mitigation table for an fda de novo submission?
-
 16:50
16:50
important new fda guidance – coming soon
-
 8:55
8:55
johnson & johnson perspective on fda draft guidance risk framework
-
 54:04
54:04
impact and implementation: understanding the new pchf analysis guidance
-
 1:04:48
1:04:48
fda | csa | csa fda draft guidance 2022
-
 49:00
49:00
csa fda guidance | csa case studies
-
 1:01:11
1:01:11
shifting requirements within the fda: expectations in chemistry study design
-
 1:28:37
1:28:37
fda cybersecurity and software policy updates: navigating the new fda guidance documents for medtech
-
 27:10
27:10
overview of post-approval chemistry, manufacture, and controls (cmc) changes to an nda - redi 2020
-
 34:02
34:02
preparing for fda remote inspections
-
 37:19
37:19
mocra draft guidance update from fda
-
 1:29
1:29
how to prepare for new fda premarket submission guidance - fda premarket submission webinar
-
 52:33
52:33
fda drug manufacturing inspections - redi 2020