oxygen is a product of the decomposition of mercury(il) oxide as shown below: 2 hgo(s) _ 2 hg() 0…
Published 1 month ago • No plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 1:12
1:12
decomposition mercury (ii) oxide and oxygen
-
 0:33
0:33
consider the decomposition of 25.0 grams of mercury (ii) oxide: 2 hgo - 2 hg o2. how many grams …
-
 0:33
0:33
mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equa…
-
 1:43
1:43
decomposition of mercury (ii) oxide : how to balance
-
 0:33
0:33
joseph priestley discovered oxygen in 1774 by heating mercury(ii) oxide. the compound decomposes in…
-
 8:50
8:50
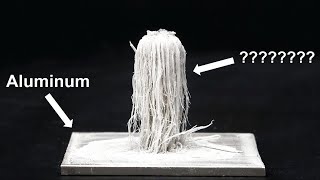
aluminum and mercury
-
 0:55
0:55
adding mercury nitrate to potasium iodide, water and silver iodide solid
-
 4:35
4:35
weekend project: how to make iodine
-
 0:33
0:33
consider the decomposition of red mercury(ii) oxide under standard state conditions. 2 hgo…
-
 0:33
0:33
the reaction of formaldehyde (g) (h2co) with oxygen (g) to form carbon dioxide (g) and water (l) pr…
-
 0:33
0:33
write out the balanced chemical equation for the thermal decomposition of mercury oxide: what did t…
-
 0:33
0:33
n2 o2 –﹥ no (reaction of nitrogen and oxygen in lightning) please balance this equation.
-
 0:33
0:33
3.8 limiting reactant; mercury(il) oxide, hgo. the british chemist joseph priestley prepared oxygen…
-
 0:33
0:33
the element oxygen was prepared by joseph priestley in 1774 by heating mercury(ii) oxide: hgo(s) ↅ
-
 0:33
0:33
in a study of the thermal decomposition of lithium peroxide, 2 li_2 o_2(s) …̊
-
 0:33
0:33
hydrogen peroxide can decompose to water and oxygen by the reaction 2 h2o2(l) ? 2 h2o(l) o2(g) ?h…
-
 0:33
0:33
the decomposition of nitrogen dioxide to nitrogen and oxygen is second order with a rate constant k…
-
 0:33
0:33
look at problem # 1 in the post lab questions the reaction described is the decomposition of diiodi…
-
 0:33
0:33
draw the product(s) of the reaction shown. draw the appropriate number of hydrogens on oxygen and n…
-
 0:33
0:33
for the gas phase decomposition of dinitrogen pentoxide at 335 k: 2 n2o5 -﹥ 4 no2 o2 the average …
-
 0:33
0:33
score on last try: 0 of 1 pts. see details for more. next question: you can retry this question bel…
-
 0:33
0:33
as stated in the chapter, potassium superoxide (ko_2) is a useful source of o…