what principle states that no two electrons in an atom can have the same set of quantum numbers?
Published 1 month ago • 342 plays • Length 0:18Download video MP4
Download video MP3
Similar videos
-
 6:00
6:00
pauli exclusion principle
-
 10:36
10:36
the basic math that explains why atoms are arranged like they are: pauli exclusion principle
-
 15:59
15:59
i never understood why electrons have spin... until now!
-
 19:29
19:29
how electron spin makes matter possible
-
 5:52
5:52
how can we test a theory of everything?
-
 5:23
5:23
the ultimate quantum physics quiz!
-
 0:14
0:14
the pauli exclusion principle simplified
-
 1:00
1:00
overview of pauli exclusion principle
-
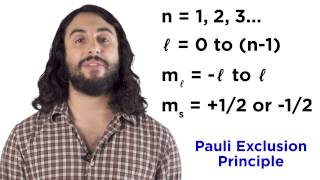 8:42
8:42
quantum numbers, atomic orbitals, and electron configurations
-
 0:55
0:55
the pauli exclusion principle: the rule that shapes atoms!
-
 0:06
0:06
quiz 299 : what does the pauli exclusion principle state ?
-
 1:47
1:47
pauli exclusion principle
-
 5:24
5:24
aufbau's principle, hund's rule & pauli's exclusion principle - electron configuration - chemistry
-
 20:52
20:52
what causes the pauli exclusion principle?
-
 0:11
0:11
pauli exclusion principle #utubeshorts
-
 8:44
8:44
pauli's exclusion principle | identical and indistinguishable particles
-
 0:48
0:48
the pauli exclusion principle and the spin quantum number (ms) - general chemistry shorts
-
 0:35
0:35
daily mcqs | physics
-
 0:56
0:56
the mysterious nature of spin
-
 12:16
12:16
quantum numbers
-
 1:41
1:41
pauli's exclusion principal
-
 0:58
0:58
pauli's exclusion principle