regulatory pathway and data requirements for ndct, 2019
Published 4 years ago • 6.1K plays • Length 43:11Download video MP4
Download video MP3
Similar videos
-
 43:58
43:58
tables given in ndct 2019 and its content
-
 44:40
44:40
salient feature of ndct 2019 - what's new in ndct?
-
 1:00
1:00
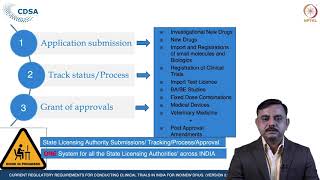
current regulatory requirements for conducting clinical trials in india - course introduction
-
 25:56
25:56
pre-clinical data requirements
-
 28:35
28:35
which biocompatibility tests do you need to do for a 510(k)?
-
 1:12:03
1:12:03
nec contracts - the risk register/early warning register and risk allocation and management
-
 27:10
27:10
online submission 23a: sugam
-
 14:56
14:56
introductory video 1
-
 6:28
6:28
nonrigid registration
-
 16:03
16:03
clinical trial related guidelines (ndct rules)
-
 2:11
2:11
introductory video 2
-
 22:32
22:32
overview of new drugs and clinical trials rules, 2019
-
 10:12
10:12
current regulatory requirements for conducting clinical trials in india - overview
-
 21:28
21:28
how do you write a regulatory pathway analysis?
-
 34:38
34:38
c1l07
-
 0:56
0:56
introductory video 1
-
 26:16
26:16
c1l10b
-
 33:16
33:16
rules governing clinical trials