silver chromate (ag2cro4; ksp 1.1x10-12) has a molar solubility of 6.5x10-5 m. what calculation pro…
Published 2 months ago • 7 plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 0:33
0:33
silver chromate is sparingly soluble in aqueous solutions. the ksp of ag2cro4 is 1.12ã—10^−12. wh…
-
 0:33
0:33
calculate the solubility of ag2cro4 (ksp = 9.0 ã— 10^–12) in a 0.054 m agno3 solution. 1.7ã—10^-1…
-
 3:26
3:26
⚗️ calculating kₛₚ from molar solubility (ag₂so₄)
-
 0:33
0:33
the ksp for an ionic compound with the formula mx2 is 3.91 x 10 6. calculate the solubility (g/l) o…
-
 0:33
0:33
what is the molar solubility of caf_2 in a 0.100-m solution of hf ? k_…
-
 2:39
2:39
silver chloride (agcl)
-
 0:45
0:45
hocl3, sncl2 🔬
-
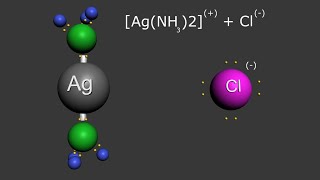 6:24
6:24
solubility of silver chloride in ammonia
-
 0:33
0:33
silver arsenate (ag3aso4) is a slightly soluble salt having a solubility product of ksp = 1.0 x 10^…
-
 0:33
0:33
calculate the molar solubility and the solubility in g/l of each salt at 25â°c: (a) pbf2 ksp = 4.0 …
-
 0:33
0:33
question 33 (2 points) if the ksp of ca(oh)2 is 6.5 x 10^-6, what is its solubility? a) 1.1 x 10^5 …
-
 0:33
0:33
at a given temperature, the solubility of ce2(so4)3 is 74.4 g/l. the molar mass of ce2(so4)3 is 568…
-
 0:33
0:33
calculate the molar solubility of co(oh)3, ksp = 2.5 x 10-43.
-
 0:33
0:33
what is the concentration of ca2 (aq) in a saturated solution of caco3? (note: the solubility produ…
-
 0:33
0:33
what is the molar solubility of iron (ii) phosphate if ksp = 1.3x10^22? 6.4x10^9 m 5.2x10^5 m 11x10…
-
 0:33
0:33
calculate the solubility of solid ca3(po4)2 (ksp=1.3ã—10^-32) in a 0.20 m na3po4 solution.
-
 0:33
0:33
4. a. give the chemical equation for the dissociation of pbcl2. b. what is the molar solubility of …
-
 0:33
0:33
the concentrations of calcium and sulfate ions in a saturated aqueous solution of caso4 are both 0.…
-
 0:33
0:33
the solubility of bacro4 (formula mass = 253) in water is 2.8 x 10-3 gl-1. what is the ksp of the s…
-
 0:33
0:33
the solubility of calcium chromate is 1.56 x 10^(-8) g/100 ml of solution. complete the following s…