the molecule in the next column is 2,2 -dimethylpropan-1-ol, a primary alcohol. because no h atoms …
Published 2 months ago • No plays • Length 0:33Download video MP4
Download video MP3
Similar videos
-
 2:05
2:05
structural formula for 2-methyl-2-butanol (tert amyl alcohol)
-
 11:50
11:50
how to draw lewis structures
-
 8:14
8:14
predict the products when 1-propanol is heated in the presence of h include all hydrogen atoms in
-
 0:59
0:59
naming an alkyl halide
-
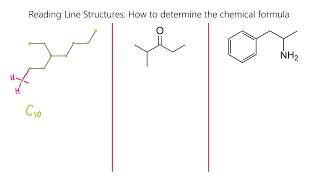 8:40
8:40
reading skeletal line structures (organic chemistry), part 1
-
 0:39
0:39
condensed structural formula to full lewis structure #organicchemistry #studywithme
-
 6:59
6:59
s3.2.5 - how do we convert between full structural, condensed, and skeletal formulas? (old 10.1)
-
 6:31
6:31
vsepr theory and molecular geometry
-
 1:01:50
1:01:50
the atmospheric physics behind net zero
-
 6:08
6:08
kekule structures and condensed structures
-
 3:23
3:23
michael donors
-
 11:29
11:29
condensed structures to skeletal structures
-
 4:41
4:41
lewis dot structures
-
 0:46
0:46
dynamic equilibrium in chemistry | explained very precisely
-
 58:55
58:55
lecture 9 alcohol, phenol, thiol, and ethers
-
 3:45
3:45
introducing a carbon takeback policy - prof. myles allen
-
 5:51
5:51
o nitrophenol is more acidic than o cresol - alcohols, phenols and ethers - chemistry class 12
-
 11:16
11:16
mole concept lecture 1
-
 5:04
5:04
9| is alcohol acidic than water? if yes why? alcohols, phenols & ethers |chemistry cbse 12
-
 0:56
0:56
the bcl3 is a planar molecule whereas ncl3 is pyramidal because
-
 3:11
3:11
halogenation and ozonolysis of alkynes
-
 11:20
11:20
acidity of ethanol and phenol