the value of pharmacopeial reference standards
Published 3 years ago • 1.5K plays • Length 35:16Download video MP4
Download video MP3
Similar videos
-
 2:05
2:05
value of a usp reference standard
-
 1:35
1:35
value beyond the vial | usp reference standards
-
 1:58
1:58
reference standards planning & development | usp
-
 2:28
2:28
usp reference standards app video
-
 1:54
1:54
technical support for reference standard users
-
 1:32
1:32
usp monograph & reference standards process
-
 3:22
3:22
what is a pharmacopeia?
-
 0:36
0:36
reference standards, types, uses, preparation & qualification
-
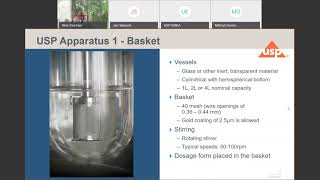 1:02:17
1:02:17
qualification of dissolution testers usp performance verification test (pvt)
-
 58:50
58:50
validation, verification, & transfer of analytical methods – usp general chapters 1224, 1225 & 1226
-
 17:12
17:12
you must know these facts about the diluted standard method for rs by hplc
-
 4:53
4:53
pharmacopeial forum (pf) alert 45(1)
-
 1:07
1:07
verifying pharmaceutical quality: standards
-
 1:06
1:06
what is united states pharmacopeia?
-
 1:39
1:39
how can quality standards help reduce the environmental impact of pharmaceutical manufacturing?
-
 0:31
0:31
standards help ensure the quality of generic medications | usp
-
 57:45
57:45
characterisation of non-compendial reference standards for impurities
-
 37:22
37:22
characterisation of non-compendial reference standards for impurities: how good is good enough?
-
 4:18
4:18
usp global education & training
-
 6:49
6:49
dr ranjan chakrabarti united states pharmacopoeia
-
 4:51
4:51
usp-nf online official status tutorial
-
 8:47
8:47
how to determine the potency of working/reference standard (dried vs anhydrous vs as such basis)