usability reports for #510(k) submissions hfe tip #fda #usability #humanfactorsengineering
Published 6 months ago • 19 plays • Length 0:48Download video MP4
Download video MP3
Similar videos
-
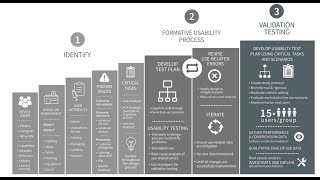 18:29
18:29
fda human factors guidance simplified
-
 43:39
43:39
leverage fda human factors engineering guidelines to enhance your r&d process
-
 1:33:59
1:33:59
510(k) project management - updated for 2021
-
 55:59
55:59
complex electronic components testing, mosfet, transistor, voltage regulator, pwm ic, opto-isolator
-
 1:57:43
1:57:43
how to diagnose faults in transistor circuits - a practical example samson txm16 1000w powered mixer
-
 53:04
53:04
how to find substitutes for discontinued transistors
-
 7:01
7:01
sys-048 usability procedure
-
 30:25
30:25
medical device usability: highlights of european regulations and the latest standards
-
 32:31
32:31
managing human factors in reprocessing of reusable devices- validation considerations
-
 49:11
49:11
human factors and quality testing for device reprocessing
-
 1:30:07
1:30:07
michael wiklund & jon tilliss : applying human factors in medical software development (nov 2015)
-
 0:50
0:50
sf5j41a electronic component
-
 1:16
1:16
microchip’s mcp48fxb family of d-to-a converters retain settings at power-down
-
 2:01
2:01
analog devices chronous™
-
 3:23
3:23
voortman v807 | changing consumables
-
 4:58
4:58
adisimrf version 1.5 by analog devices
-
 5:41
5:41
power supply input derating explained 💡