which solution has the highest vapor pressure a 20 0 g of glucosec6h12o6 in 100 0 ml of water b 20
Published 4 months ago • 16 plays • Length 4:35
Download video MP4
Download video MP3
Similar videos
-
 0:33
0:33
which solution has the highest vapor pressure? a. 20.0 g of glucose(c6h12o6) in 100.0 ml of water b…
-
 4:03
4:03
which has the highest vapor pressure? 20 g c6h12o6 glucose, 20gc12h22o11 sucrose 10 g kc2h3o2 p
-
 1:54
1:54
vapor pressure and boiling
-
 25:23
25:23
colligative properties - boiling point elevation, freezing point depression & osmotic pressure
-
 5:44
5:44
vapor pressure. how to use vapor pressure of solution to find the amount of solute added?
-
 55:46
55:46
intermolecular forces and trends, formal charges, hund's rule, lattice structures and unit cells
-
 10:40
10:40
intermolecular forces - hydrogen bonding, dipole dipole interactions - boiling point & solubility
-
 5:37
5:37
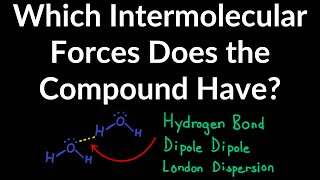
how to identify the intermolecular force a compound has: london dispersion, dipole dipole, h-bonding
-
 2:05
2:05
the vapour pressure of water at 20°c is 17.5mmhg. if 18g of glucose (c6h12o6 ) is
-
 10:54
10:54
intermolecular forces and boiling points
-
 3:05
3:05
an ordinary glass is filled to the brim with 450 0 ml of water at 100 0c if the temperature of glas
-
 12:46
12:46
predicting relative boiling point, vapor pressure, and solubility in water based on imf
-
 21:14
21:14
07 ch 12 vapor pressure curves
-
 4:27
4:27
chemistry of gases (29 of 40) boiling point and water vapor pressure
-
 6:16
6:16
chemistry - liquids and solids (49.5 of 59) phase change: vapor pressure & partial pressure
-
 6:00
6:00
ranking vapor pressures
-
 0:33
0:33
what is the vapor pressure of water at point e ?
-
 2:46
2:46
the vapour pressure of water at 20°c is 17.5mm hg.if 18gm of glucose is added to 178.2g of water.
-
 4:37
4:37
which molecules have higher (or lower) vapor pressure
-
 3:00
3:00
the vapour pressure of water at`20^@c`is `17.5mmhg`.if `18g`of glucose
-
 45:36
45:36
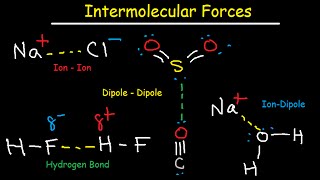
intermolecular forces - hydrogen bonding, dipole-dipole, ion-dipole, london dispersion interactions
-
 1:16
1:16
18 g of glucose (c6h12o6) is added to 178.2g of water. the vapour pressure of water for this aqu....
Clip.africa.com - Privacy-policy
 0:33
0:33
 4:03
4:03
 1:54
1:54
 25:23
25:23
 5:44
5:44
 55:46
55:46
 10:40
10:40
 5:37
5:37
 2:05
2:05
 10:54
10:54
 3:05
3:05
 12:46
12:46
 21:14
21:14
 4:27
4:27
 6:16
6:16
 6:00
6:00
 0:33
0:33
 2:46
2:46
 4:37
4:37
 3:00
3:00
 45:36
45:36
 1:16
1:16