calculate the solubility of agcl in 0.1 m nacl at 25°c if its solubility product at same tem....
Published 1 month ago • 32 plays • Length 1:41Download video MP4
Download video MP3
Similar videos
-
 1:09
1:09
calculate solubility of agcl in 0.1 m nacl at `25^(@)c` if its solubility proudct at same
-
 4:52
4:52
worked example: calculating solubility from kₛₚ | equilibrium | ap chemistry | khan academy
-
 9:57
9:57
15.15a | calculate the concentration of all solute species: agcl(s) in 0.025 m nacl
-
 2:11
2:11
what happens to the solubility of agcl in water if nacl solution is added is to ? | 12 | ionic e...
-
 2:09
2:09
if the solubility of `agcl` in `0.1 m` nacl is (`k_(sp)` of `agcl = 1.2 xx 10^(-10)`)
-
 3:51
3:51
calculation of solubility.
-
 8:36
8:36
solubility product constant (ksp)
-
 2:22
2:22
agcl hno3 = ??? (no reaction)
-
 3:01
3:01
the solubility of agcl in 0.1 molar nacl
-
 4:29
4:29
the solubility of a solution of agci(s) with solubility product 1.6 xx 10^(-10) in 0.1 m nacl so...
-
 1:56
1:56
calculate the solubility of \( \mathrm{agcl} \) at \( 373 \mathrm{~k} \). the solubility product...
-
 0:36
0:36
the solubility of agcl(s) with solubility product 1.6x 10-10 in 0.1 m nacl solution would be
-
 8:18
8:18
calculate the molar solubility of \( \mathrm{agcl} \) in a 1-l solution which contains \( 10.0 \...
-
 1:55
1:55
the solubility product of agcl in water at 25^∘c is 1.6 × 10^-10. its solubility in the presence ...
-
 0:33
0:33
calculate the molar solubility of agcl in 0.0100 m nacl solution: ksp of agcl 1.77 x 10-10 1.33 x 1…
-
 41:52
41:52
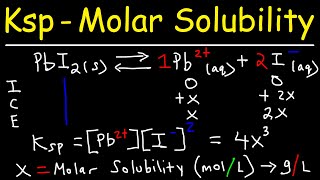
ksp - molar solubility, ice tables, & common ion effect
-
 4:20
4:20
solubility product of `agcl` is `2.8xx10^(-10)` at `25^(@)c`. calculate solubility of the salt
-
 2:27
2:27
the solubility of agcl in 0.2 m nacl solution (k_(sp) for agcl = 1.20 xx 10^(-10)) is | class 1...
-
 1:55
1:55
the solubility of `agcl` in `0.1 m nacl` is (`k_(sp)` of `agcl = 1.2 xx 10^(-10)`)
-
 12:38
12:38
equilibrium: the solubility product
-
 4:11
4:11
what happens to the solubility of `agcl` in water if `nacl` solution is added is to ?
-
 7:42
7:42
let the solubilities of `agcl` in pure water be `0.01 m cacl_(2)`, `0.01 m nacl` and