the solubility of agcl in 0.1 molar nacl
Published 3 years ago • 922 plays • Length 3:01Download video MP4
Download video MP3
Similar videos
-
 2:09
2:09
if the solubility of `agcl` in `0.1 m` nacl is (`k_(sp)` of `agcl = 1.2 xx 10^(-10)`)
-
 1:55
1:55
the solubility of `agcl` in `0.1 m nacl` is (`k_(sp)` of `agcl = 1.2 xx 10^(-10)`)
-
 1:41
1:41
calculate the solubility of agcl in 0.1 m nacl at 25°c if its solubility product at same tem....
-
 2:27
2:27
the solubility of agcl in 0.2 m nacl solution (k_(sp) for agcl = 1.20 xx 10^(-10)) is | class 1...
-
 2:15
2:15
the solubility of agcl is `1xx10^(-5)mol//l`. its solubility in 0.1 molar sodium chloride
-
 0:33
0:33
calculate the molar solubility of agcl in 0.0100 m nacl solution: ksp of agcl 1.77 x 10-10 1.33 x 1…
-
 4:29
4:29
the solubility of a solution of agci(s) with solubility product 1.6 xx 10^(-10) in 0.1 m nacl so...
-
 33:21
33:21
buffer solutions
-
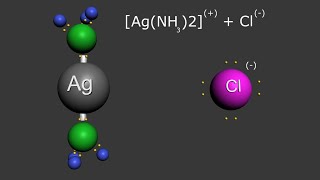 6:24
6:24
solubility of silver chloride in ammonia
-
 53:22
53:22
chemical equilibrium constant k - ice tables - kp and kc
-
 8:18
8:18
calculate the molar solubility of \( \mathrm{agcl} \) in a 1-l solution which contains \( 10.0 \...
-
 1:09
1:09
calculate solubility of agcl in 0.1 m nacl at `25^(@)c` if its solubility proudct at same
-
 2:11
2:11
what happens to the solubility of agcl in water if nacl solution is added is to ? | 12 | ionic e...
-
 0:36
0:36
the solubility of agcl(s) with solubility product 1.6x 10-10 in 0.1 m nacl solution would be
-
 5:13
5:13
what is the molar solubility of agcl(s) in 0.1 m nh_(3)(aq)?k_(sp)(agcl)=1.8xx10^(-10), k_(f)[ag...
-
 4:07
4:07
solubility of \( \mathrm{agcl} \) in water, \( 0.01 \mathrm{m} \mat...
-
 8:08
8:08
find the solubility of `agcl` in `0.1 m cacl_(2)`. `e^(c-)._(ag^(o )|ag)=0.799v` and that of `agcl|
-
 4:11
4:11
what happens to the solubility of `agcl` in water if `nacl` solution is added is to ?
-
 41:52
41:52
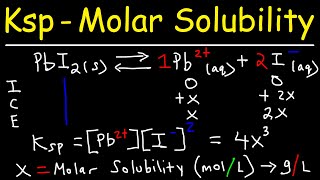
ksp - molar solubility, ice tables, & common ion effect
-
 0:33
0:33
a. what is the solubility of silver chloride (agcl) in a solution of pure water at 25â°c, if the so…
-
 0:33
0:33
isotonic saline solution is 0.154 m nacl(aq). what is the solubility of agcl (ksp = 1.8 ã— 10^-10) …
-
 12:38
12:38
equilibrium: the solubility product