conducting medical device clinical investigations in switzerland
Published 9 years ago • 106 plays • Length 2:38Download video MP4
Download video MP3
Similar videos
-
 4:39
4:39
conducting medical device clinical investigations in switzerland
-
 2:57
2:57
conducting medical device clinical investigations in germany
-
 1:50
1:50
conducting medical device clinical investigations in singapore
-
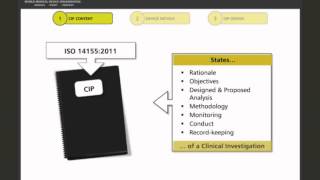 2:53
2:53
format and structure of a medical device clinical investigation plan/protocol
-
 51:27
51:27
webinar: clinical investigations - transitioning from mdd to mdr
-
 2:27
2:27
what are the tasks of swissmedic in the area of medical devices?
-
 51:27
51:27
webinar: clinical investigations - transitioning from mdd to mdr
-
 19:47
19:47
common interview questions in pharmacovigilance
-
 58:41
58:41
navigating the global ivd landscape
-
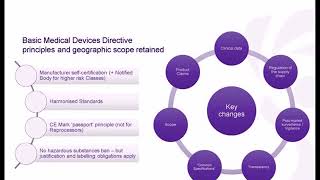 1:06:28
1:06:28
medical devices regulation training
-
 4:49
4:49
regulatory challenges on medical device studies - challenges in clinical research
-
 10:48
10:48
medical device searching, part 1: new eu regulations and the role of literature
-
 4:00
4:00
the responsibilities of a clinical investigation sponsor
-
 1:29
1:29
medical device clinical trials
-
 3:00
3:00
european safety reporting requirements during pre-market clinical investigations
-
 21:25
21:25
ihuk 2020 - clinical validation and regulatory landscape for ai medical devices
-
 2:39
2:39
cpm: medical device clinical trials - budget and timelines planning
-
 1:04:01
1:04:01
bc seminar series: medical device regulation - a reality check. by dr. daniel delfosse
-
 36:29
36:29
clinical evaluation of medical devices according to eu mdr 2017 745
-
 2:50
2:50
gcp for investigators: how to qualify for medical device clinical investigations
-
 0:26
0:26
6. imds: conduct medical device clinical trials based on iso14155