how many orbitals in an atom can have the designation? 5p 3dz2 4d n=5 n=4
Published 3 years ago • 2.9K plays • Length 5:28Download video MP4
Download video MP3
Similar videos
-
 7:38
7:38
how many orbitals in an atom can have each of the following designations a 5f b 4p c 5d d n = 2
-
 2:23
2:23
how many orbitals are in the n=4 shell?
-
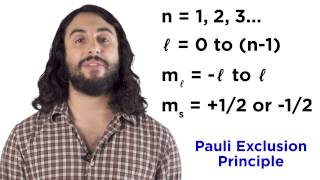 8:42
8:42
quantum numbers, atomic orbitals, and electron configurations
-
 9:23
9:23
shells, subshells, and orbitals - biology/chemistry ep5
-
 3:46
3:46
how many orbitals are there in n=3, the 3rd energy level of an atom?
-
 4:44
4:44
how many orbitals in n = 4?
-
 8:39
8:39
atomic orbitals, visualized dynamically
-
 5:50
5:50
atomic orbitals 3d
-
 3:14
3:14
demonstration of spin 1/2
-
 0:22
0:22
atom 3d model orbital spin
-
 3:13
3:13
the order of filling of electrons in the orbitals of an atom will be(a) 3d, 4s, 4p, 4d, 5s
-
 11:19
11:19
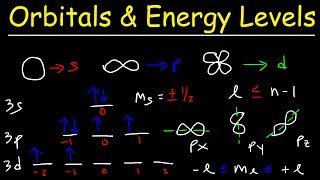
orbitals, atomic energy levels, & sublevels explained - basic introduction to quantum numbers
-
 10:55
10:55
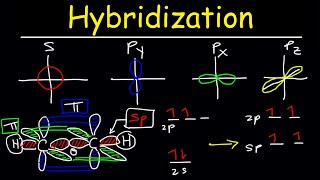
hybridization of atomic orbitals - sigma & pi bonds - sp sp2 sp3
-
 0:24
0:24
latest image of an atom! 🔬
-
 1:40
1:40
how many orbitals are in the 'p' sublevel ?
-
 12:01
12:01
spdf orbitals explained - 4 quantum numbers, electron configuration, & orbital diagrams
-
 0:30
0:30
zooming into a water 💧
-
 1:38
1:38
electron orbitals - s,p & d
-
 0:33
0:33
how many orbitals in an atom could have these sets of quantum numbers? orbital(s) orbital(s) n = 5,…
-
 12:57
12:57
how many electrons in an atom could have these sets of quantum numbers
-
 8:04
8:04
quantum theory: atomic orbitals: determine the number of subshells and give the designation when n=5
-
 3:05
3:05
quantum numbers - with designation examples