how many electrons in an atom could have these sets of quantum numbers
Published 4 years ago • 1.5K plays • Length 12:57Download video MP4
Download video MP3
Similar videos
-
 11:46
11:46
how to determine the maximum number of electrons using allowed quantum numbers - 8 cases
-
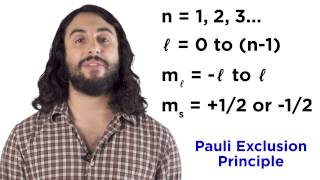 8:42
8:42
quantum numbers, atomic orbitals, and electron configurations
-
 7:20
7:20
quantum numbers - how many electrons and orbitals have the following set of quantum numbers?
-
 7:38
7:38
how many orbitals in an atom can have each of the following designations a 5f b 4p c 5d d n = 2
-
 3:14
3:14
demonstration of spin 1/2
-
 5:50
5:50
atomic orbitals 3d
-
 5:35
5:35
a better way to picture atoms
-
 2:58
2:58
how many electrons are present in a atom having quantum numbers n=3, l=1?
-
 4:03
4:03
identify which sets of quantum numbers are valid for an electron. each set is ordered (n,ℓ,mℓ,ms).
-
 6:43
6:43
how many electrons in an atom with atomic number 105 can have (n l) = 8?
-
 6:44
6:44
quantum numbers for mult-electron atoms
-
 14:17
14:17
how to determine the maximum number of electrons given a set of quantum numbers
-
 5:28
5:28
how many orbitals in an atom can have the designation? 5p 3dz2 4d n=5 n=4
-
 7:50
7:50
11std , no two electrons in an atom can have all four set of quantum numbers equal.
-
 8:43
8:43
6.66 | write a set of quantum numbers for each of the electrons with an n of 3 in a sc atom.
-
 0:48
0:48
how small are atoms?
-
 6:05
6:05
quantum numbers - worked problem| structure of atom | chemistry | khan academy
-
 10:43
10:43
quantum numbers, how to find max number of electrons in a given set? | #5
-
 0:46
0:46
let's practice: quantum numbers | atomic structure
-
 11:00
11:00
6.45 | write a set of quantum numbers for each of the electrons with an n of 4 in a se atom.
-
 10:32
10:32
electron orbitals, principle quantum number and hund's rule | radiology physics course #2
-
 4:35
4:35
why do the quantum numbers matter? (most honest explanation!)