industry-fda interactions - straight talk with susan: key issues in medical technology regulation
Published 4 years ago • 141 plays • Length 3:01Download video MP4
Download video MP3
Similar videos
-
 2:13
2:13
fda's mission - straight talk with susan: key issues in medical technology regulation
-
 1:35
1:35
risk and medical technology - straight talk with susan: key issues in medical technology regulation
-
 3:13
3:13
post-market device issues - straight talk with susan: key issues in medical technology regulation
-
 1:24
1:24
the 510k process - straight talk with susan: key issues in medical technology regulation
-
 2:44
2:44
regulation of medtech vs drugs - straight talk with susan: key issues in medtech regulation
-
 1:43
1:43
clinical trials and medtech - straight talk with susan: key issues in medical technology regulation
-
 2:10
2:10
patient information - straight talk with susan: key issues in medical technology regulation
-
 2:08
2:08
iteracare device testimonial...
-
 56:55
56:55
fda process for medical device startups: an investor's point of view
-
 1:28:00
1:28:00
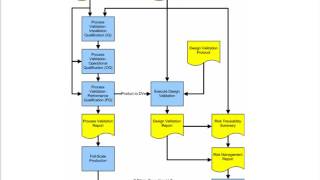
process validation for medical device manufacturers
-
 1:43
1:43
510(k) clinical testing - straight talk with susan: key issues in medical technology regulation
-
 56:46
56:46
medical technology-enabled data platforms: ip, fda and compliance issues
-
 1:00:01
1:00:01
fda regulation of medical devices - abridged and simplified
-
 57:47
57:47
fda 101 for medical devices
-
 54:13
54:13
114 - discover strategies for de-risking medical device development: fda consensus standards
-
 16:18
16:18
how is my medical device classified?
-
 6:50
6:50
overview of the usa fda classification process
-
 6:42
6:42
dr. scott gottlieb: the challenge with a.i. tools is getting it regulated by fda as medical devices
-
 17:43
17:43
case study: how is my medical device classified?