regulatory framework in iso 11607
Published 4 weeks ago • 6 plays • Length 6:18Download video MP4
Download video MP3
Similar videos
-
 5:58
5:58
sterile barrier systems in iso 11607
-
 6:45
6:45
reusable sterile barrier systems in iso 11607
-
 3:57
3:57
introduction to iso 11607 : packaging for terminally sterilized medical devices
-
 4:44
4:44
key definitions and terminology in iso 11607
-
 6:08
6:08
record-keeping best practices in iso 11607
-
 22:56
22:56
iso 11607 packaging changes explained | 10x medical device conference
-
 5:39
5:39
design requirements in iso 11607
-
 1:28:00
1:28:00
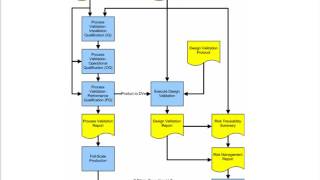
process validation for medical device manufacturers
-
 1:08:18
1:08:18
sterility validation 101: ensuring a robust sterilization validation program from start to finish
-
 1:20:03
1:20:03
iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes
-
 6:01
6:01
environmental considerations in iso 11607
-
 6:15
6:15
corrective and preventive actions (capa) in iso 11607
-
 1:00:51
1:00:51
navigating packaging changes in light of new regulatory requirements
-
 30:34
30:34
regulatory requirements for packaging changes
-
 8:39
8:39
class a medical devices regulatory framework enhancements part 1 of 4
-
 1:01:22
1:01:22
packaging validation of medical devices - impact of the revisions of iso 11607 & suitable strategies
-
 6:44
6:44
class a medical devices regulatory framework enhancements part 4 of 4
-
 8:39
8:39
overview of the current medical device regulatory framework in singapore part 1 of 2
-
 57:56
57:56
medical device package validation: review and updates on standardized test methods of iso 11607
-
 7:20
7:20
class a medical devices regulatory framework enhancements part 3 of 4