which of the following orbital does not exist according to quantum theory? (a) 5 g (b) 4 f (c) 5...
Published 2 years ago • 57 plays • Length 1:54Download video MP4
Download video MP3
Similar videos
-
 2:41
2:41
, which atomic orbital will not exist according to quantum theory?(a) 4 f (b) 6 g (c) 7 d (d) 5...
-
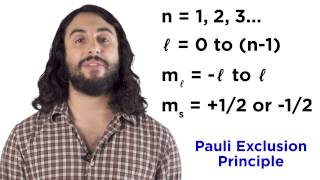 8:42
8:42
quantum numbers, atomic orbitals, and electron configurations
-
 6:04
6:04
which of the following orbitals can not exist?
-
 2:37
2:37
which of the following subshell(s) do(es) not exist for an atom, according to quantum theory? (a...
-
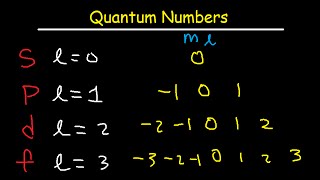 4:25
4:25
how to determine the 4 quantum numbers from an element or a valence electron
-
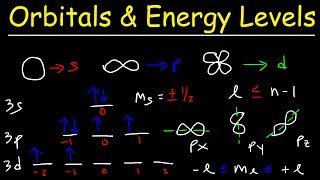 11:19
11:19
orbitals, atomic energy levels, & sublevels explained - basic introduction to quantum numbers
-
 12:16
12:16
quantum numbers
-
 9:08
9:08
changes everything: new research suggests that gravity can exist without mass
-
 10:19
10:19
michio kaku explains the mysteries of string theory & quantum physics
-
 42:47
42:47
why everything you thought you knew about quantum physics is different - with philip ball
-
 4:47
4:47
two weeks of chemistry in 5 minutes...
-
 37:50
37:50
chem 101 ch7 quantum theory part 3 ismail badran
-
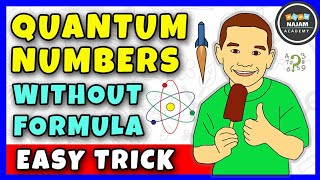 12:10
12:10
quantum numbers | what are the 4 quantum numbers? chemistry
-
 0:45
0:45
is 3f orbital possible?
-
 10:24
10:24
planck's quantum theory | chemistry
-
 8:04
8:04
quantum theory: atomic orbitals: determine the number of subshells and give the designation when n=5
-
 2:19
2:19
which of the following is not correct according to planck\'s quantum theory ?
-
 0:15
0:15
if you think you understand quantum mechanics, then you don’t understand quantum mechanics
-
 1:22
1:22
brian cox explains quantum mechanics in 60 seconds - bbc news
-
 1:01
1:01
plank's quantum theory #chemistry #quantum #natueal #shorts #trending #trendingshorts #youtube
-
 1:22:07
1:22:07
development of quantum theory
-
 7:20
7:20
quantum numbers - how many electrons and orbitals have the following set of quantum numbers?