which of the following orbitals can not exist?
Published 2 years ago • 3.7K plays • Length 6:04
Download video MP4
Download video MP3
Similar videos
-
 2:14
2:14
which of the following orbitals does not have angular node?
-
 6:09
6:09
which combination of quantum numbers is not allowed?
-
 3:54
3:54
which of the following sets of numbers can describe a 3p electron?
-
 2:05
2:05
which of the following orbitals can not undergo hybridisation ? (i) \( 3 d, 4 s \) (ii) \( 3 d, ...
-
 11:19
11:19
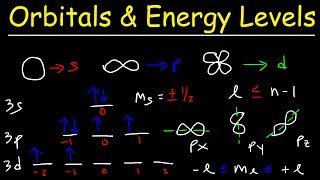
orbitals, atomic energy levels, & sublevels explained - basic introduction to quantum numbers
-
 14:46
14:46
quantum physics of endothermic and exothermic reactions simplified!
-
 8:39
8:39
atomic orbitals, visualized dynamically
-
 5:35
5:35
a better way to picture atoms
-
 3:28
3:28
which of the following orbitals does not participate in the hybridisation in `if_(7)` ?
-
 9:23
9:23
shells, subshells, and orbitals - biology/chemistry ep5
-
 2:00
2:00
which of the following orbitals are possible ? `1pm, 2s, 3f, 3d`
-
 1:11
1:11
which combinations of n and l represent real orbitals, and which do not exist a 1s b 2p c 4s d 2
-
 1:17
1:17
which of the following orbital does not same ?
-
 1:54
1:54
which of the following orbital does not exist according to quantum theory? (a) 5 g (b) 4 f (c) 5...
-
 11:46
11:46
how to determine the maximum number of electrons using allowed quantum numbers - 8 cases
-
 3:01
3:01
f-orbitals cannot be present in which of the following shell ? n=1,n=2,n=3,n=4,n=5 hint : \'l\' ...
-
 1:16
1:16
which of the following orbital does not same ?
-
 4:34
4:34
which of the following orbitals are possible? 1p, 2s, 2p and 3f
-
 2:53
2:53
which of the following sub-shell does not exist for an atom, according to quantum theory ?
-
 3:18
3:18
identifying the shapes of the orbitals
-
 3:10
3:10
which of the following combinations of n and l represent real orbitals and which are impossible? 2s,
-
 1:42
1:42
which of the following pairs of d-orbitals will have electron density along the axes?(a) dz2, dxz
Clip.africa.com - Privacy-policy
 2:14
2:14
 6:09
6:09
 3:54
3:54
 2:05
2:05
 11:19
11:19
 14:46
14:46
 8:39
8:39
 5:35
5:35
 3:28
3:28
 9:23
9:23
 2:00
2:00
 1:11
1:11
 1:17
1:17
 1:54
1:54
 11:46
11:46
 3:01
3:01
 1:16
1:16
 4:34
4:34
 2:53
2:53
 3:18
3:18
 3:10
3:10
 1:42
1:42